Chem131 - General Chemistry I
1/13
Earn XP
Description and Tags
Key terms and concepts needed to pass
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
John Dalton’s Theory of the Atom
1) Atoms are indestructible particles that make elements (REFUTED)
2) atoms of one element cannot change to other atoms of another element (REFUTED)
3) all atoms of an element have the same mass and properties (REFUTED)
4) atoms can bind in integers to form compounds
Refutes
1) Atoms can be broken down into protons, electrons, and neutrons
2) atoms can be isotopes, asotopes, or Ions, which don’t have the same mass
3) atoms can change into other atoms through radioactivity
Subatomic particles
Protons —> 1.67262 × 10^-27 —> +1 charge
Neutrons —> 1.67493 × 10^-27 —> 0 charge
Electrons —> 0.00091 × 10^-27 —> -1 charge
Protons
abbreviation = p+
~ identity of element
~ found by atomic # (z)
~ if two atoms have same # of protons, it is the same element
Neutrons
abbreviation = n0
~ keep atoms stable
~ too many protons or neutrons, an atom can shed a proton, changing its identity
~ acts as a buffer between protons in the nucleus
Electrons
abbreviation = e-
~ drives reactivity
~ Same # of electrons = same # of protons
~ if perfect amount of electrons = no bonds created
~ if perfect number is not achieved = react by gaining or losing electrons
whichever requires less energy
lose electron = positive charge = cations
gain electron = negative charge = anions
Isotopes
two atoms with same number of protons but different number of neutrons
ex. C-12 & C-14 (carbon and carbon dating), CO-60 & CO-59 (radiation therapy)
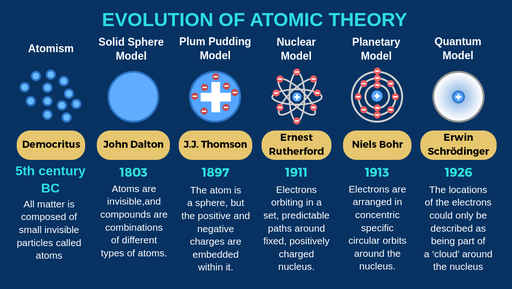
Atomic models throughout the years
Dalton’s Theory of Atoms
Plum Pudding - an atom contains small negatively charged particles called electrons, and electrons exist in a positively charged space
Nuclear model of the atom - 1) Most of the atom’s mass and all of its positive charge are contained in a small core (nucleus). 2) The volume of the atom is empty, throughout which tiny negatively charged electrons are dispersed. 3) Many negatively charged electrons outside the nucleus are positively charged protons in the nucleus —> keep the atom electrically neutral
Bohr model - nucleus remains the same, electrons are in concentric orbits at a fixed distance from the nucleus
energy is represented as concentric rings; specific levels —> discrete
electron releases energy when transitioning from a higher to a lower energy
seen as photons (light energy)
Quantum Model - nucleus remains same, electrons don’t orbit but exist in probability dense domain that predicts positions
exist in all areas at the same time, we must measure its positions exist in order to see it in that moment
Electrons exist as waves until measured, then they become particles
Quantum numbers
n = principle quantum number (energy level)
l = angular momentum quantum number (orbital type)
Ml = magnetic quantum number (which orbital) —> s,p,d,f
Ms = magnetic spin quantum number
Pauli Exclusion Principle
no two electrons can have the same 4 QNs
Coulomb’s law
charge and repulsion
particle of like charges repel (energy lowers when they separate)
allowing like charges to occupy space at a distance
particles of unlike charges attract (energy lowers when closer together)
gets funny because all the atoms want to be near nucleus
larger the charges, larger the energetic effect
bigger charge = stronger energy attraction = lower energy position
Shielding and Effective Nuclear charge
total amount of attraction an electron feels for nucleus’s protons
the electrons want be as close to the nucleus as possible
Penetration
the shape of orbitals allow electrons the possibility to get closer to the nucleus, which lowers its energy overall
Aufbau Principle
electrons enter atomic orbitals from lowest energy to highest
Hund’s rule
electrons will always enter empty orbital before they pair up