13.3/ WM 1- Alcohols
1/143
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
144 Terms
Name 9 industrial uses of alcohols?
· Thermometers,
· fuel (internal combustion engines),
· solvents,
· alcoholic beverages (ethanol),
· antifreeze (ethylene glycol),
· medicines,
· antiseptic,
· preservative for specimens,
· hand sanitizer.
What is the purpose of a medicine?
When something is wrong with the body medicines help to prevent it becoming worse and can provide a cure
Name two examples of medicines?
Penicillin/ Aspirin
What are the active ingredients of medicines?
Drugs- substances that alter the way your body works
When would a drug in a medicine not be beneficial?
When your body is already working normally
What is a poison?
A drug which throws your body a long way off balance
Are all drugs medicines?
No- e.g. alcohol and nicotine
Some may or may not be medicines depending on your state of health
How was the effectiveness of drugs first discovered?
Trial and error- sometimes with bad mistakes
Why is it easier to make medicines today?
Today medicines are designed to have specific effects
Easier to create medicines as scientists learn more about the body's chemistry and begin to understand the intricate detail of the complex molecules from which people are made- this understanding is from the study of molecular pharmacology
What is pharmacology/ a pharmacy?
Pharmacology- study of drugs and their actions
Pharmacy- making and dispensing medicines
Where did medicines originally come from?
History of medicine- herbal and folk remedies (Many of these can be explained in present day terms)Modern pharmaceutical industries were built off folklore remedies- today they investigate “old wives tales” to see if they lead to important new medicines
Name and explain 3 examples of traditional medicines?
Feverfew- has been used since ancient times for the treatment of migraines, research in the 1970s showed that it was an effective medicine for migraines
Foxglove- contains compound digitalin which is active against heart disease
Willow Tree - Extracts from willow trees have been used in medicines for thousands of years, aspirin originally from willow tree bark (did not know it was aspirin at the time)
400 BC- Hippocrates recommended a brew of willow leaves to reduce the pain of childbirth
1793- Reverend Edward Stone- English clergyman living in Chipping Norton, Oxfordshire used a willow bark tree brew to reduce fevers
What does willow bark actually contain?
Unknown at the time the substance salicin which was extracted from willow bark has no pharmaceutical effect by itself but the body converts it by hydrolysis and oxidation to salicylic acid (it can be oxidised because it contains a primary alcohol group)
What is the functional group of an alcohol?
-OH (hydroxyl)
What is the general formula of an alcohol?
CnH2n+1OH
What property do all alcohol molecules have, why?
Molecules are polar because of the polarised O-H bond
What sort of bonding occurs between alcohol molecules, what does this effect?
Hydrogen bonding between the -OH groups of neighbouring molecules influences physical properties
What is the relative strength of hydrogen bonds?
Hydrogen bonds not as strong as covalent bonds but are the strongest intermolecular forces stronger than other attractive forced between covalent molecules
Draw a diagram of a hydrogen bond?
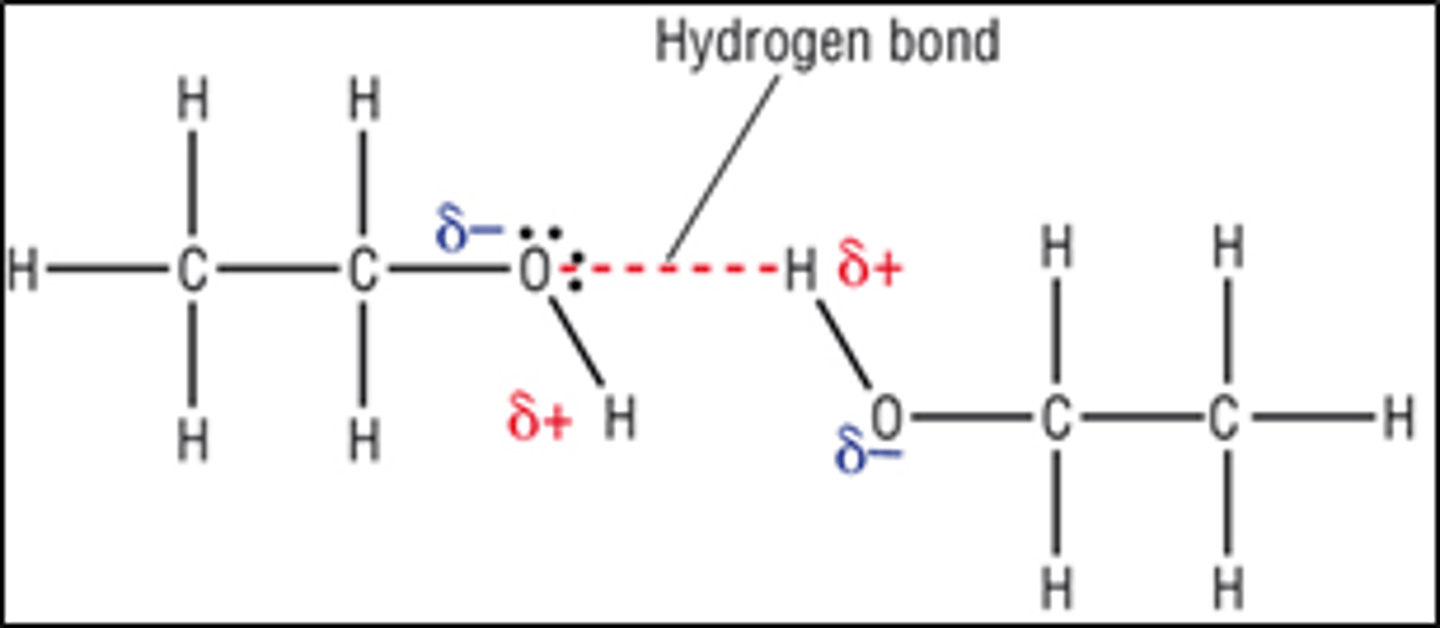
How do the general properties of the alcohols change as chain length increases, why?
As the hydrocarbon chain becomes longer and the molecule becomes larger then influence of the -OH group on the properties of the molecules becomes less important
So the properties of the higher alcohol get more and more like the corresponding alkane
What three properties of alcohols does hydrogen bonding lead to?
High melting and boiling points
Soluble in water
Relatively low volatility
Describe the effect of hydrogen bonding on the melting and boiling point of alcohols, how does the melting and boiling point of alcohols compare to alkanes with a similar Mr?
H bonding leads to relatively high melting and boiling points of alcohols compared to corresponding alkanes with similar Mr
Alcohol is usually liquid at room temp while alkanes with a similar Mr are gases
H bonds must be broken to allow the liquid to turn to a gas so more energy must be supplied to overcome these hydrogen bonds compared to corresponding alkanes which only have Van der Waals’ forces which are relatively easy to overcome so require less heat energy so have lower melting and boiling points
Why is an alcohol molecule able to dissolve in water?
Alcohols dissolve in water because H bonds form between the polar -OH groups of the alcohol and the polar O-H bonds in the water molecules
How does chain length affect the solubility of an alcohol in water, describe the solubility of primary alcohols?
Solubility decreases as chain length increases- larger part of the alcohol molecule is made up of a non-polar hydrocarbon chain, hydrocarbon chain cannot form hydrogen bonds with water molecules (only non-polar C-H bonds). Larger it gets more of the molecule cannot associate with water- less soluble
The solubility of primary alcohols in water decreases (methanol, ethanol, propan-1-ol all fully soluble (first three members of the homologous series), after that all the alcohols with the -OH group on their first carbon get less and less soluble)
Draw a diagram to explain why alcohols are soluble in water?
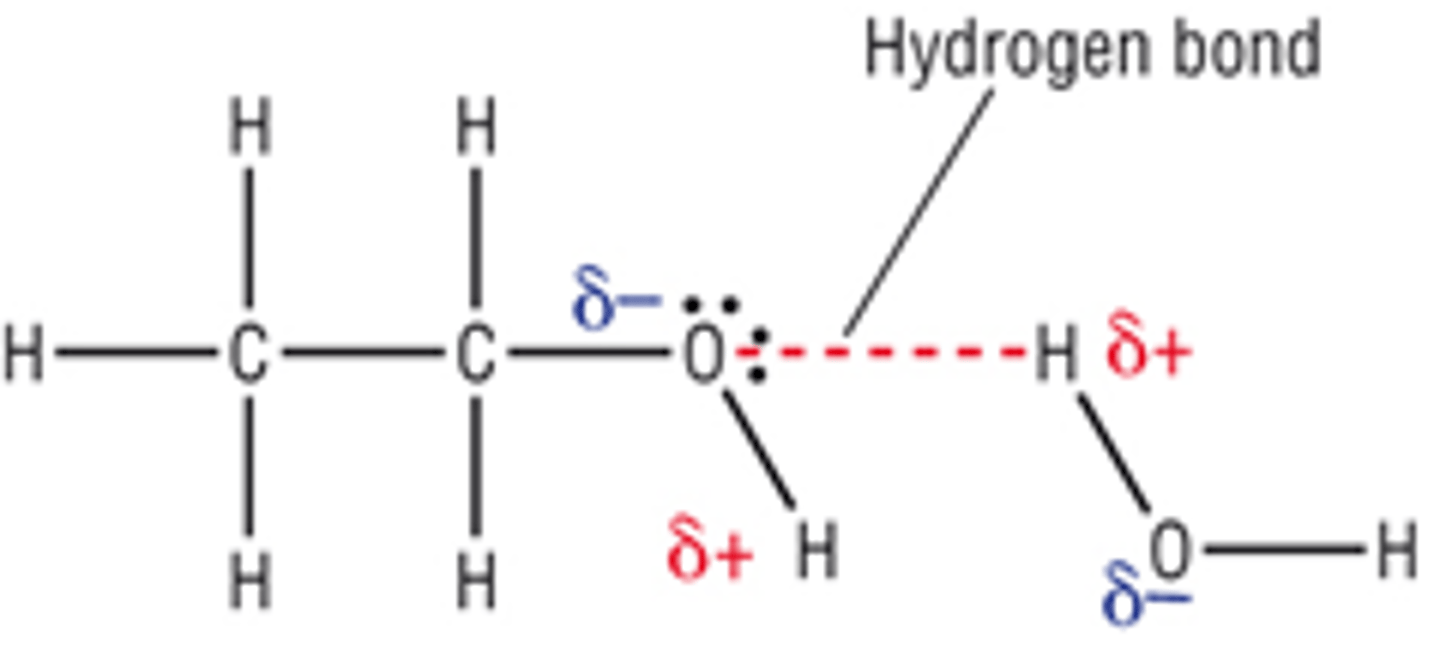
What is volatility?
The ease that a liquid turns into a gas, volatility increases as boiling point increases. A volatile chemical evaporates readily into the atmosphere at room temp/pressure
Describe and explain the relative volatility of alcohols?
Very similar explanation to mp/bp
H bonds between alcohol molecules means there is a lower volatility than alkanes of a similar molecular mass- evaporate less easily
Describe what happens to the volume of the solution if ethanol and water are mixes, explain why?
50 cm3 of ethanol and 50cm3 of water- makes 98cm3 of solution, hydrogen bonds form between the water and ethanol molecules drawing the molecules closer together than in pure ethanol/ pure water reducing volume
What is generated as hydrogen bonds form between water and alcohol molecules?
Heat
What three things do you need to remember when drawing hydrogen bonds?
1. Dotted lines to show hydrogen bonds
2. Delta sign (the +/-)- the dipole
3. Lone pairs on the oxygen- hydrogen bonds
ALWAYS go on the lone pair
What are the three types of alcohol?
Primary, secondary and tertiary- affects the types of reactions
What structural feature decides if an alcohol is primary, secondary or tertiary?
Named according to the position of the -OH group/ number of alkyl groups attached to the carbon carrying the alcohol group
EXAM TIP: what must you remember to draw in for the full structural/ displayed formula of an alcohol?
Must include the O-H bond (line between O and H not just OH)
What word is used to describe any compound containing a C=O bond?
A carbonyl compound
Aldehydes, ketones are carbonyl compounds with a polar double bond (C=O)
What are the only two carbonyl compounds
Aldehydes and ketones
Is the C=O bond polar or non-polar?
Polar as there is a large difference in electronegativity between oxygen and carbon (oxygen is a lot more electronegative than carbon)
Describe the structure of a primary alcohol:
1) The positions of the -OH group
2)The number of alkyl groups attached to the carbon with the -OH group
3) The general formula
4) An example
5) The symbol used for a primary alcohol
1)-OH bonded to carbon bonded to one other carbon atom
Attached to a carbon atom with no alkyl groups or only bonded to one alkyl group
2) 1/none
3) RCH2OH
4) butan-1-ol
5) 1^0
Describe the structure of a secondary alcohol:
1) The positions of the -OH group
2)The number of alkyl groups attached to the carbon with the -OH group
3) The general formula
4) An example
5) The symbol used for a secondary alcohol
1) -OH bonded to carbon bonded to two other carbon atom/ two other alkyl groups
2) 2
3) RCH(OH)R
4) butan-2-ol
5) 2^0
Describe the structure of a tertiary alcohol:
1) The positions of the -OH group
2)The number of alkyl groups attached to the carbon with the -OH group
3) The general formula
4) An example
5) The symbol used for a tertiary alcohol
1) -OH bonded to carbon bonded to three other carbon atom/ three alkyl groups
2) 3
3) R2C(OH)R
4) 2-methlypropan-2-ol
5) 3^0
Which two chemical processes can be used to make ethanol?
(Catalytic) Hydration of ethene or fermentation
EXAM TIP: If asked to compare the two processes for ethanol production what points could you make?
Compare the:
1) Reagents
3) Conditions
3) Equations for both
4) Advantaged and disadvantages of each process- think about the finite/ renewable resources, energy requirements, atom economy
What scale production is the catalytic hydration of ethene used for when producing ethanol?
This method is used for industrial scale production of ethanol e.g. for fuel or as a chemical in industry
Catalytic hydration of ethene: What type of reaction is the ?
Reaction is reversible- conversion of the ethene is incomplete
Catalytic Hydration of Ethene: Describe the method
Steam and ethene passed over a catalyst
Gases then have to be cooled to turn the ethanol into liquid
Catalytic Hydration of Ethene: What are the reactants, catalyst and conditions?
Reactants- steam and ethene gas
Catalyst- Phosphoric (V) acid catalyst (H3PO4)
Conditions- Temperature: 300^oC, Pressure: 60-70 atmospheres
Catalytic Hydration of Ethene: What percentage conversion to ethanol is achieved each time?
Each time the reagents pass through the reactor only 5% of the ethene is converted into ethanol- gases are recycled and passed through the reactor again
Overall a 95% conversion is achieved
Catalytic Hydration of Ethene: What is the equation for this process?
CH2=CH2 (g)+ H2O(g) ⇌ CH3CH2OH(g)
H3PO4, 300oC, 60-70atm- over the arrow
Catalytic Hydration of Ethene: Name 4 advantages of this process compared to fermentation?
1. Very fast reaction, can quickly produce lots of ethanol
2. Production is continuous
3. 100% atom economy but a low yield
4. Much higher percentage of ethanol- does not need distilling which saves money/ energy
Catalytic Hydration of Ethene: Name 4 disadvantages of this process compared to fermentation?
1. Uses finite resources/non-renewable (ethene is from crude oil fractional distillation)
2. Low yield (only 5% each time)
3. Lots of energy needed- expensive due to high temperature and pressure conditions
4. Need specialist equipment
What scale of production is fermentation used for when producing ethanol?
Small scale manufacture (such as the alcohol beverage industry)
Fermentation: What is the reaction type?
Fungi anaerobic respiration
Fermentation: Describe the method?
Carbohydrates are converted into ethanol and carbon dioxide
Ethanol solution produced like this has a concentration of up to 14% alcohol by volume
Fermentation: What percentage conversion is achieved each time?
Ethanol solution produced like this has a concentration of up to 14% alcohol by volume or 15%???
Toxicity of alcohol also limits the concentration of the ethanol that can be made by fermentation- enzymes are denatures and can no longer catalyse the reaction above 14% alcohol concentration
Fermentation: What are the reactants, catalyst and conditions?
Reactants: Carbohydrate source- sugar (cane)/ starch
Water
Catalyst: Yeast- an enzyme in the yeast called zymase is the catalyst (biological catalyst)
Conditions:
Pressure: Normal
Temperature: 25-37oC- Reaction is slow at temperatures below 25 degrees C. Above 37 degrees C the enzymes (zymase) start to denature and lose their efficiency
Solution: sealed (no air)- anaerobic conditions- no oxygen so that the yeast respire anaerobically to produce ethanol. Anaerobic conditions prevent the oxidation of ethanol to undesirable compounds such as ethanal/ ethanoic acid- would affect the flavour of the product.
Fermentation: What is the equation for this reaction?
C6H12O6 (aq)→ 2CH3CH2OH(aq) + 2CO2 (g)
25-37oC, Yeast- over the arrow
Fermentation: Name 3 advantages of this process compared to the catalytic hydration of ethene?
1. Renewable resources- sugar cane (glucose), yeast and water
2. Low temperature- Little energy reduces costs
3. Carbon neutral- plant grows, takes in carbon dioxide, released again- balanced
Fermentation: Name 5 disadvantages of this process compared to the catalytic hydration of ethene?
1. Batch production- more time consuming
2. Production rate is slow
3. Product is impure (only 15%) needs more distillation/ processing to increase purity which- expensive, lots of energy
4. Carbon dioxide produced
5. Lower atom economy- carbon dioxide is also produced
Uses of Ethanol: What are the two categories of alcohol beverage that ethanol can be used to produce?
1. Low Percentage- made by fermentation, brewery- barely, hops, yeast to make beer/ lager, fermented sugar from barley grains, hops give taste, hops prevent the action of bacteria- could spoil the beer making process
2. Spirits- made in a distillery, higher alcohol percentage, fermented drink is distilled by slowly heating the alcohol-water mixture, alcohols boils off faster than water, then it is allowed to condense, distillate then has a higher alcohols percentage than the original liquid
Name some uses of ethanol?
Alcoholic beverages, perfumes, aftershaves and cleaning fluids, solvent in methylated spirits, potential alternative for petrol
Describe the use of ethanol as a solvent in methylated spirits?
Ethanol mixed with a bit of methanol and coloured dye, methanol and dye make it toxic (undrinkable), exempt from tax so much cheaper than the ethanol in alcoholic drinks, used as a solvent for removing paint, ink stains from clothing, fuel in spirit burners/ camp stoves
Describe the use of ethanol as a potential alternative for petrol?
Being developed as an alternative for petrol to fuel cars- Up to 10% ethanol is blended by petroleum to increase the octane rating of the fuel, makes the fuel burn more cleanly, ethanol based fuels from renewable resources, benefit economy and environment
Describe some of the uses of methanol?
Cheap burning fuel
Additive in high performance racing cars
Tastes and smells like ethanol- but it is extremely toxic, ingestion of small amounts can lead to organ damage or death, can make you blind
An important feedstock for the chemical industry
Easily converted to methanal and ethanoic acid
What two different ways can alcohol combust, what conditions are needed for each?
Complete combustion in excess oxygen
Incomplete combustion in limited oxygen supply
What colour flame does ethanol burn when completely combusted?
Smokeless blue flame
What is the general word equation for the complete combustion of alcohol?
Alcohol + oxygen (excess supply)→ water + carbon dioxide
Give the symbol equation for the complete combustion of ethanol?
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l)
What two factors do the products of alcohol oxidation depend on?
Reaction conditions (reflux), type of alcohol used
Name the oxidation products of primary, secondary and tertiary alcohols
Primary:
First oxidation- ALDEHYDE
Second oxidation- CARBOXYLIC ACID (then it is completely oxidised)
Secondary:
First oxidation- KETONE (then it is completely oxidised)
No second oxidation
Tertiary:
Not oxidised
What happens when an alcohol is oxidised, what is needed, what is oxidised and what is reduced?
The -OH group of the alcohol can be oxidised by strong oxidising agents such as acidified potassium dichromate(VI)- K2Cr2O7 solution
The -OH group is converted to a carbonyl C=O group
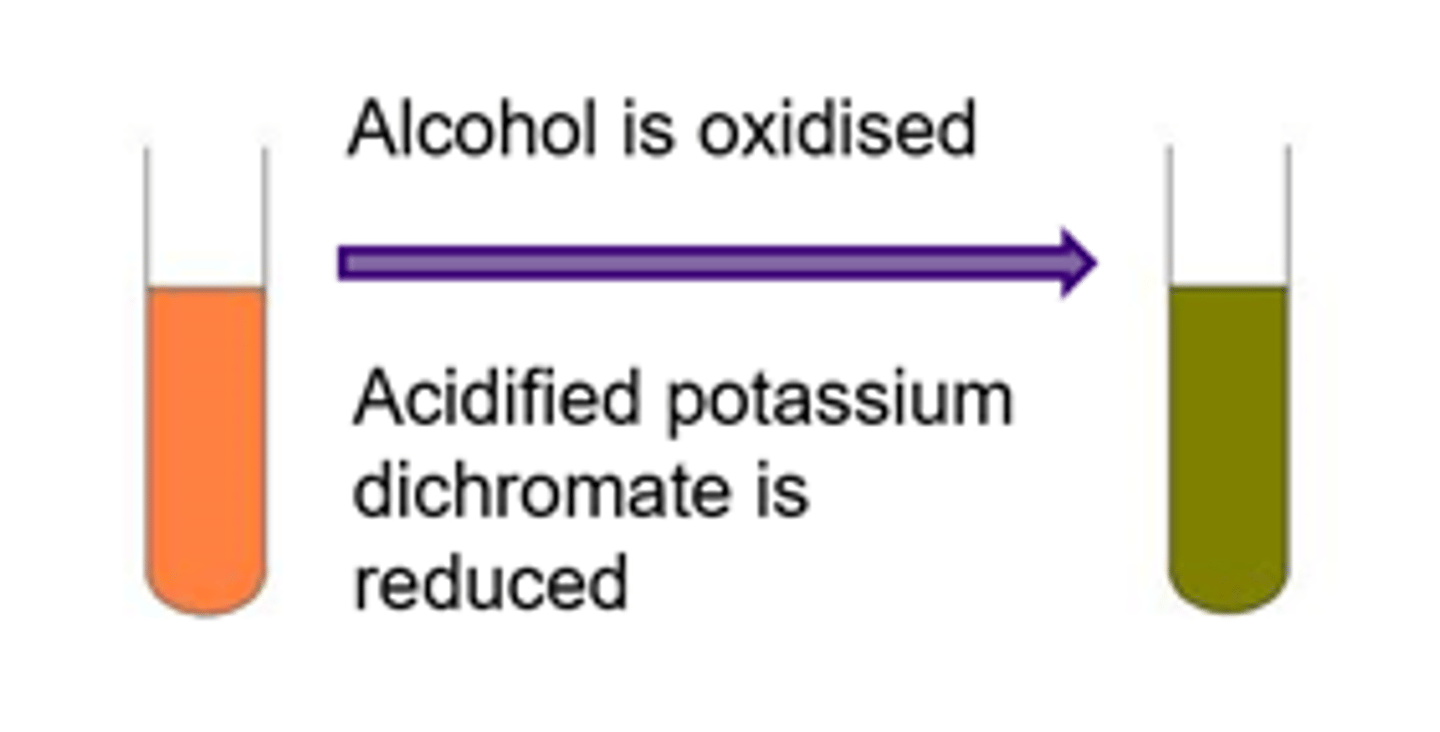
Alcohol is oxidised, acidified potassium dichromate is reduced
In this reaction two atoms of hydrogen are being removed- one from the oxygen atom and one from the carbon atom
Exam Question: What observation can be made when an alcohol is oxidised?
Orange dichromate (VI) ion Cr2O72-(aq) is reduced to green chromate (III) ions Cr3+(aq) in the reaction- the reaction mixture turns from orange to green
What structure is needed to allow the alcohol to be oxidised?
Oxidation of the -OH group will not take place unless there is a hydrogen atom on the carbon atom to which the -OH is attached- which is why tertiary alcohols cannot be oxidised like this
What is needed in the solution for an alcohol to be oxidised?
An oxidising agent
Name some suitable oxidising agents for the oxidation of an alcohol, how is it made?
A solution containing acidified dichromate ions H+/Cr2O72-
EXAM TIP; Cr2O72-/H+ does not actually exist so when asked for an example of an oxidising agent it must be K2Cr2O7(aq)/ H+(aq)- acidified potassium dichromate (VI)
Made from potassium dichromate and sulphuric acid
Can use a different metal as long as it is acidified
EXAM TIP: What symbol is used to show an oxidising agent in a reaction?
[O] is used to show the oxidising agent
First oxidation of a primary alcohol: what is the product, what conditions are needed, describe the method, what is the colour change, draw a diagram of the apparatus?
Product- ALDEHYDE
Conditions- Gentle heating, acidified potassium dichromate (no excess oxidising agent, no reflux)
Method- When preparing an aldehyde in the lab
Once the aldehyde is formed there will be lots of oxidising agent remaining to turn it into a carboxylic acid, need to get the aldehyde out of the oxidising solution as fast as possible to prevent it being further oxidised to a carboxylic acid- do this with distillation apparatus- distil aldehyde off immediately
The aldehyde boils at a lower temperature than the alcohol so it evaporates and is distilled off immediately
Colour Change- Orange dichromate (VI) ion Cr2O72-(aq) is reduced to green chromate (III) ions Cr3+(aq) in the reaction- the reaction mixture turns from orange to green
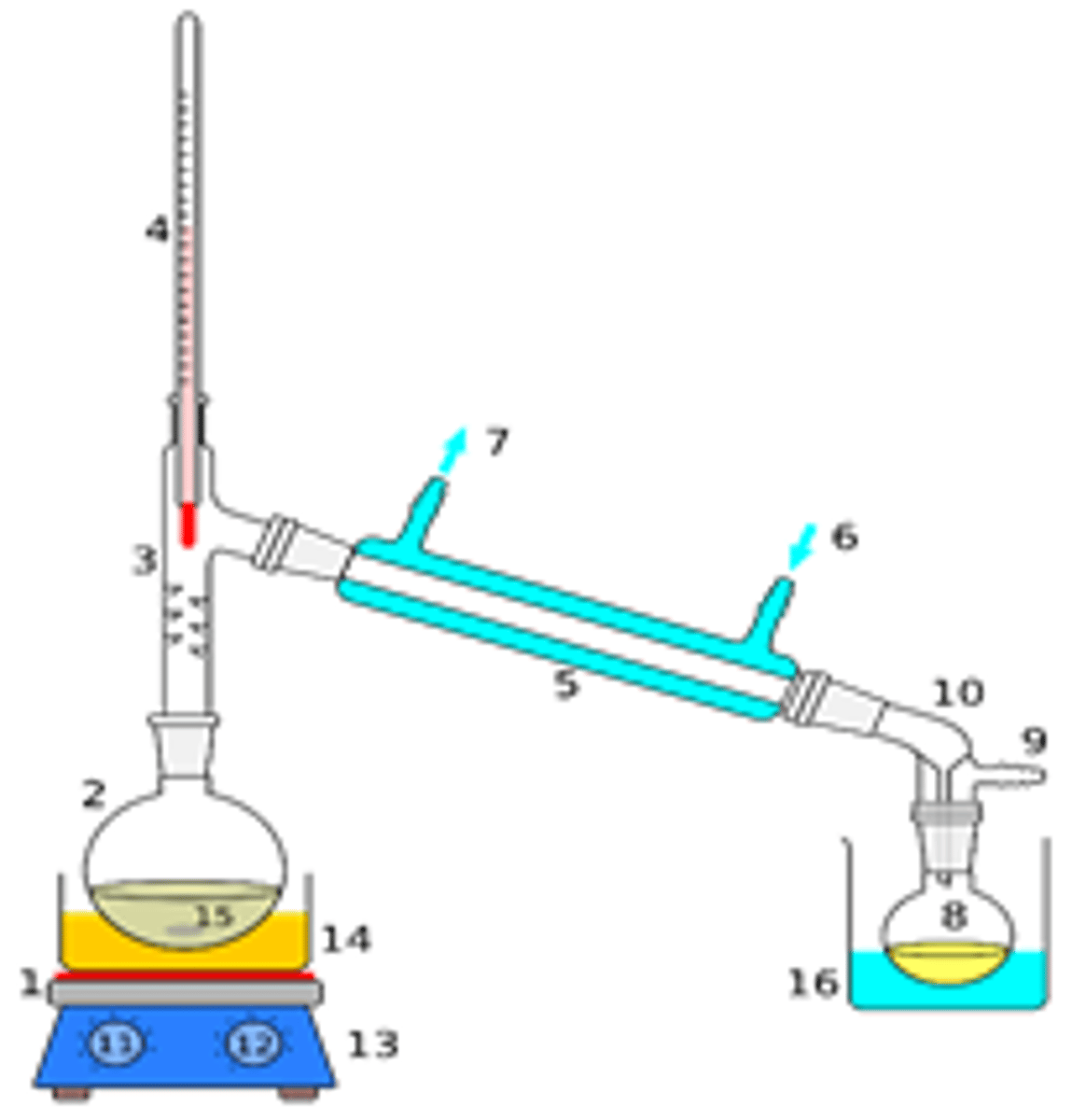
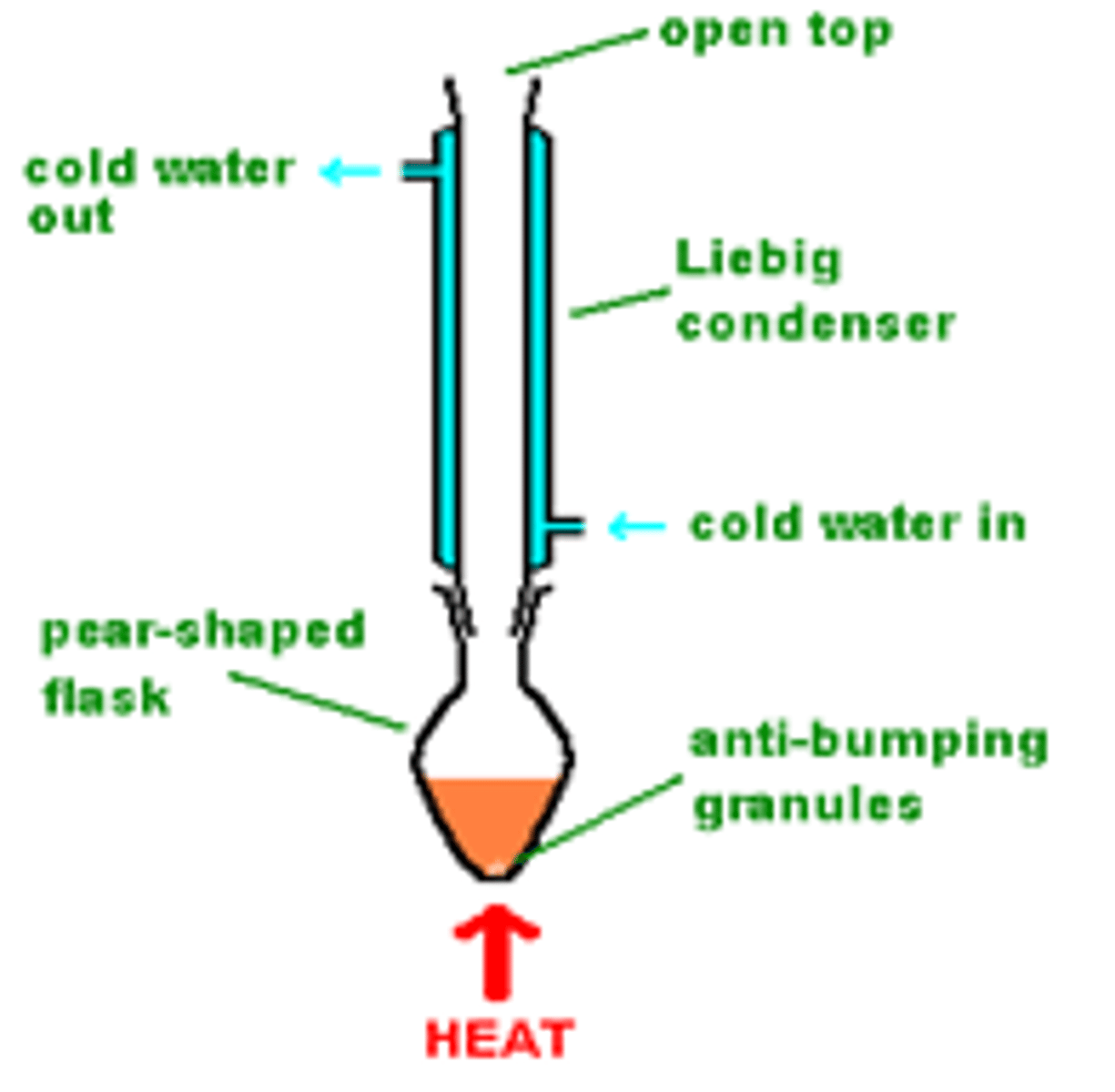
EXAM/ PRACTICAL TIP: for the simple distillation of an aldehyde during the first oxidation of a primary alcohol which way around must the condenser be connected and why?
Water should flow in the bottom of the condenser and out the top
If you connect the condenser the wrong way around, the water cannot fill the outer water jacket of the condenser and will not cool the water vapour effectively so the water must always enter lower than it leaves
Draw the displayed formula for the first oxidation of the primary alcohol propan-1-ol
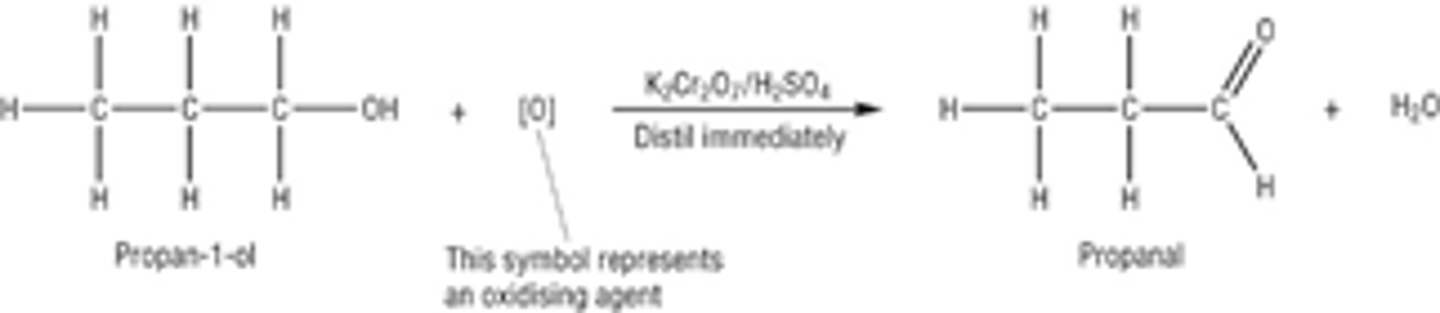
Secondary oxidation of a primary alcohol: what is the product, what conditions are needed, describe the method, what is the colour change, draw a diagram of the apparatus used?
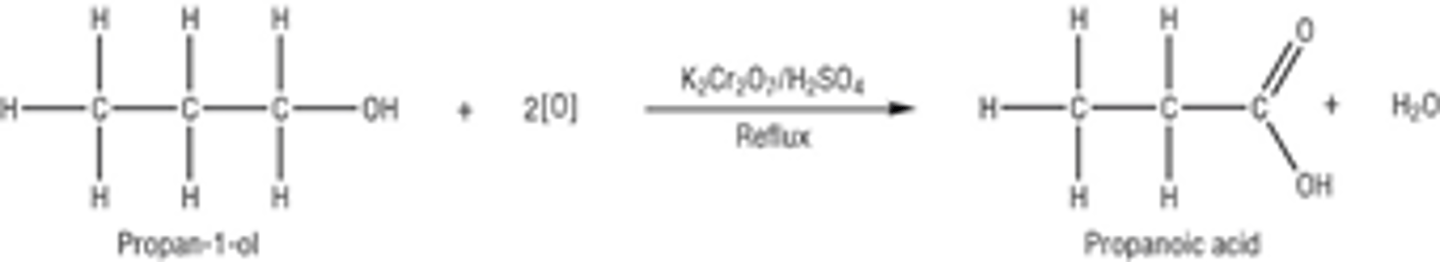
Product- CARBOXYLIC ACID
The aldehyde is then oxidised to a carboxylic acid Conditions- in the presence of excess oxidising agent and stronger heating
Method- heat the reaction mixture under reflux before distilling off the product
The alcohol is completely oxidised, passing through the aldehyde stage to form a carboxylic acid
Colour Change- Orange dichromate (VI) ion Cr2O72-(aq) is reduced to green chromate (III) ions Cr3+(aq) in the reaction- the reaction mixture turns from orange to green
Define reflux
The continual boiling and condensing of a reaction mixture to ensure that the reaction takes place without the contents of the flask boiling dry
For what two reasons would you use a reflux technique when oxidising a primary alcohol to a carboxylic acid?
1. So you can increase the temperature of the organic solvent to boiling point without losing any of the volatile solvents, reactants, products- any vaporised compounds cooled so they condense and drip back into the reaction mixture. Also safer to heat a volatile or flammable solvents this way.
2. Reflux maybe to overcome activation energy for second oxidation
Draw the displayed formula for the second oxidation of the primary alcohol propan-1-ol
What is the test to confirm that the product of the second oxidation of an alcohol was a carboxylic acid?
Carboxylic acid test- blue/ universal litmus paper (goes red), sodium carbonate (produces carbon dioxide)
EXAM TIP: How could C4H9OH be oxidised in different ways?
Structural isomers of C4H9OH that are alcohols may be primary, secondary, tertiary and so may have different oxidation products
May ask in an exam for the different structural isomers and the the products formed when each isomer is heated with excess acidified potassium dichromate
Remember the primary alcohol with the formula C4H9OH will have two oxidation products one for the first and one for the second oxidation.
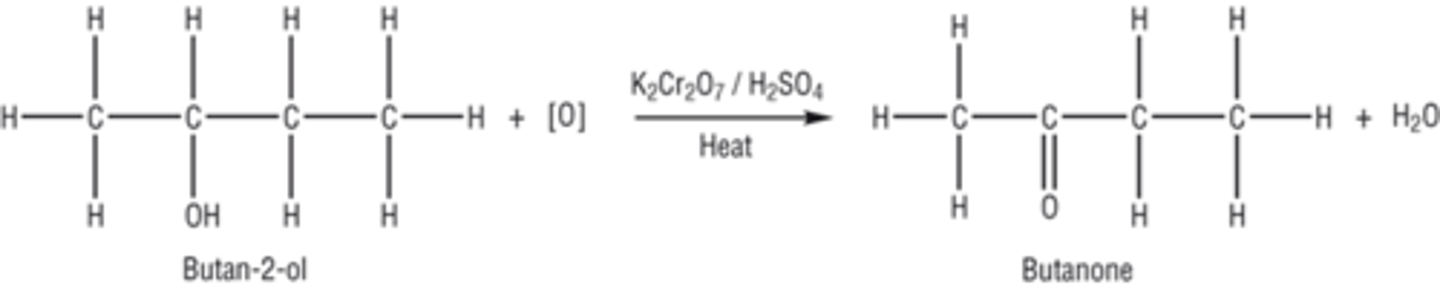
Oxidation of a secondary alcohol: what are the products and conditions needed?
Products- a ketone and water
Conditions- Heating under reflux in the presence of an oxidising agent
Why can't a ketone be oxidised further?
Ketone cannot be oxidised further even with prolonged refluxing as the strong covalent C-C bonds would need to be broken
Draw the displayed formula for the oxidation of the secondary alcohol butan-2-ol?
Why can't tertiary alcohols be oxidised, what observation would be made?
Tertiary Alcohols cannot be oxidised because they do not have a hydrogen atom on the carbon atom to which the -OH group is attached. Say they are resistant to oxidisation.
Undergo no reaction with acidified dichromate ions – the reaction mixture/ oxidising agent remains orange.
Describe the process of refluxing, what is added to the mixture?
Liquid is boiled with a vertically mounted condenser so the vapour condenses and returns back into the reaction mixture
Add anti-bumping granules- prevent bubbling over by bursting the bubbles formed- doesn't boil over
What is the general formula of a carboxylic acid?
ROOH
CnH2n+1COOH
What can be found in both aldehydes and ketones?
A carbonyl (C=O) group
Where is the C=O group found in an aldehyde?
At the end of the chain
Where is the C=O group found in a ketone?
Within the alkane chain
How do you name an aldehyde?
Suffix -al for aldehydes e.g. ethanal
How do you name a ketone?
Suffix -one e.g. propanone
What is the general formula of an aldehyde?
RCHO
CnH2n+1CHO
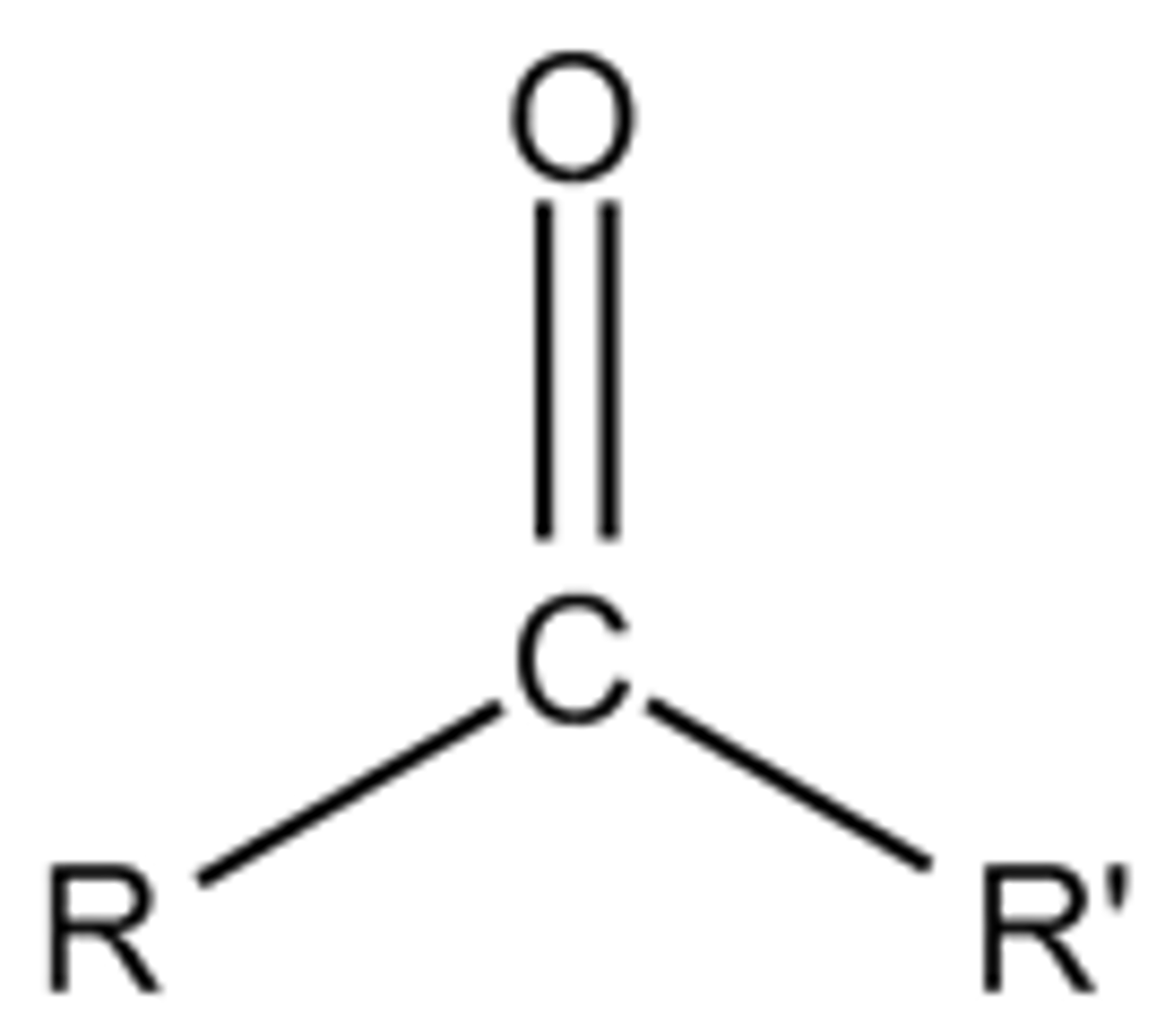
What is the general formula of a ketone?
R (C=O) R′
Draw the functional group of an aldehyde?
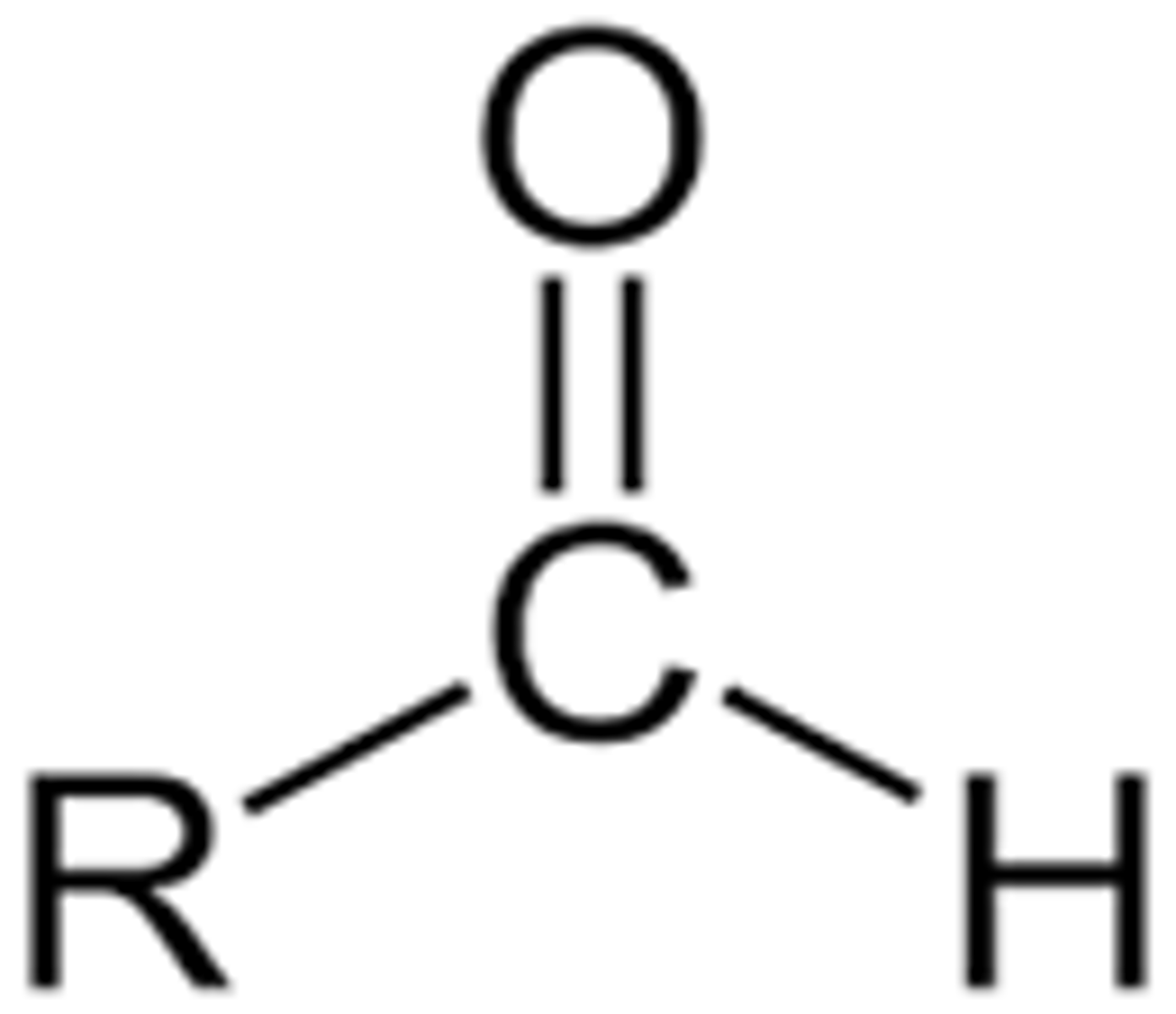
Draw the functional group of a ketone?
Define dehydration?
An elimination reaction in which water is removed from a saturated molecules to make an unsaturated molecule
Describe an alcohol dehydration reaction?
Many alcohols can be dehydrated- lose a molecule of water to form an alkene in the presence of an acid catalyst