[O Chem] Arenes
1/21
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Benzene —> Aniline
Electrophilic substitution (nitration)
Conc. H2SO4, conc. HNO3, heat under reflux at 55ºC
In the nitration of benzene, H2SO4 acts as a
Bronsted-Lowry Acid Catalyst (H+ donor)
In the nitration of benzene, HNO3 acts as a
Bronsted-Lowry Base (H+ acceptor)
Formation of stronger electrophile from HNO3
HNO3 + 2H2SO4 → NO2+ + 2HSO4- + H3O+
Benzene —> Chlorobenzene
Electrophilic substitution (halogenation)
Cl2, anhydrous FeCl3/AlCl3/Fe, room temp.
In the chlorination of benzene, FeCl3/AlCl3 acts as a
Lewis acid catalyst (e pair acceptor)
Formation of stronger electrophile from FeCl3
Cl2 + FeCl3 —> Cl+ + FeCl4-
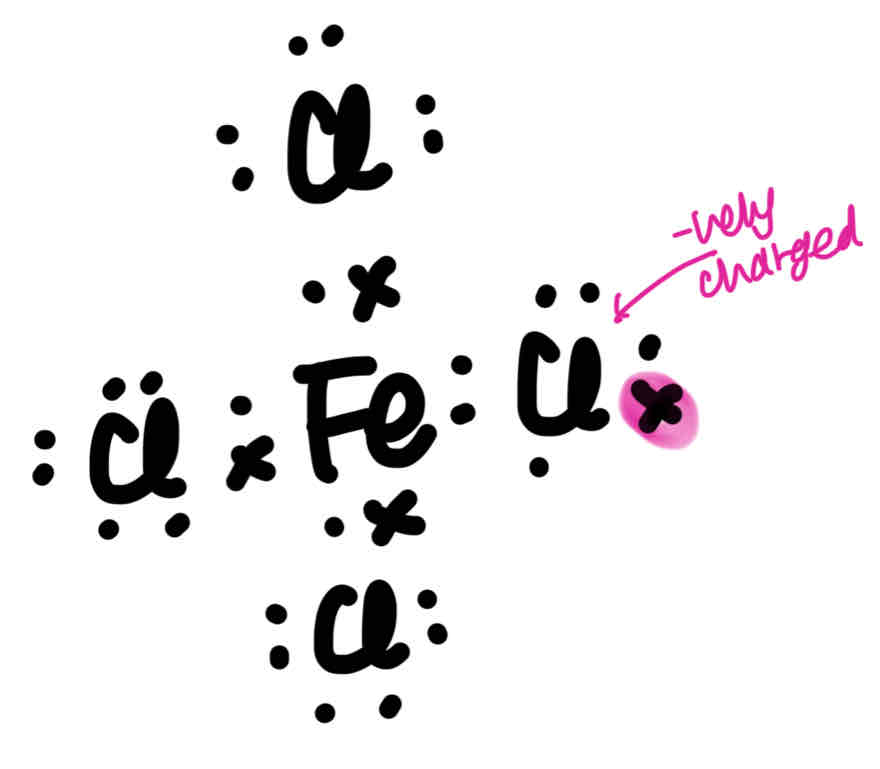
Draw the Lewis structure for FeCl4-
Toluene —> Chlorotoluene
Electrophilic substitution
Cl2, anhydrous FeCl3/AlCl3/Fe
Observations for chlorination and bromination of benzene
Yellowish-green Cl2/Reddish-brown Br2 decolourises
White fumes of HCl/HBr produced
Toluene —> Nitrotoluene
Electrophilic substitution
Conc. H2SO4, conc. HNO3, 30ºC
For polysubstitution of nitro group onto toluene, how would the R&Cs change?
heat at temperatures higher than 30ºC
Toluene —> Chloromethylbenzene
Free radical substitution
(limited/excess) Cl2 in UV light
Toluene —> Benzoic acid
Oxidation
KMnO4 in dilute H2SO4, heat under reflux
KMnO4 in KOH, room temp.
Distinguish between benzene and toluene
Add KMnO4 in H2SO4, heat in water bath
Toluene: decolourises purple KMnO4
Benzene: KMnO4 remains purple
Distinguish between toluene and longer chain alkylbenzene
Add KMnO4 in H2SO4, heat in water bath
Both decolourise purple KMnO4
Toluene: no effervescence
Alkylbenzene: effervescence of CO2 (g) which forms white ppt. with limewater
Observations for strong oxidation of toluene
Purple KMnO4 decolourises
White precipitate of benzoic acid observed
Observations for mild oxidation of toluene
Purple KMnO4 decolourises
Brown-black precipitate of MnO2 formed
Why does mild oxidation of toluene not give white precipitate?
Benzoic acid is neutralised to form benzoate ions in the alkaline medium
Freidel-Crafts Alkylation of Benzene
Electrophilic substitution
RX, anhydrous FeX3/AlX3, room temp
Observations for Freidel-Crafts Alkylation of Benzene
white fumes of HX produced
Benzene —> cyclohexane
Reduction
H2 gas, Ni cat, high temp and pressure