Classification of Matter
1/15
Earn XP
Description and Tags
These flashcards cover key vocabulary terms and concepts related to the classification of matter discussed in the lecture.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|

16 Terms
What is Matter?
Anything that takes up space and has mass.
Substance
A type of matter that has a fixed composition; all particles are identical.
Mixture
A combination of two or more substances that are physically combined with variable composition.
Element
The simplest form of matter, consisting of identical atoms, found on the Periodic Table.
Compound
A substance formed when two or more elements are chemically combined in a fixed proportion.
Homogeneous Mixture
A mixture where components are evenly distributed and appear blended, like solutions.
Heterogeneous Mixture
A mixture where components are not evenly distributed and can be seen separately, like salad dressing.
Difference between Element and Compound
An element is a single substance; a compound is multiple elements chemically combined.
Water (H2O) is formed from hydrogen and oxygen elements in a fixed proportion.
Example of a Compound
Salt water, because it has a uniform composition.
Example of a Homogeneous Mixture
Salad dressing, as the components can be seen separately.
Example of a Heterogeneous Mixture
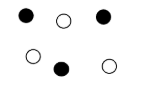
Mixture of Elements
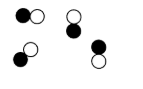
Compounds (pure substance)
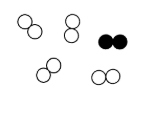
Mixture of Elements
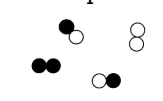
Mixture of Elements and compounds

Elements (pure substances)