Science Y10 Exam 2 (copy)
1/131
Earn XP
Description and Tags
this is so long i am so sorry
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
132 Terms
DNA
Also known as deoxyribonucleic-acid, the basis of inheritance which is made up of nucleotides and is found in the nucleus of all cells that have one. Is in the shape of a double-sided helix
Function of DNA
Stores genetic information through the complimentary base pairing rule, and helps to pass genetic information
Structure of DNA
Made up of two sugar-phosphate backbones (the vertical lines), connected by lines of nitrogenous bases connected by hydrogen bonds (the horizontal)
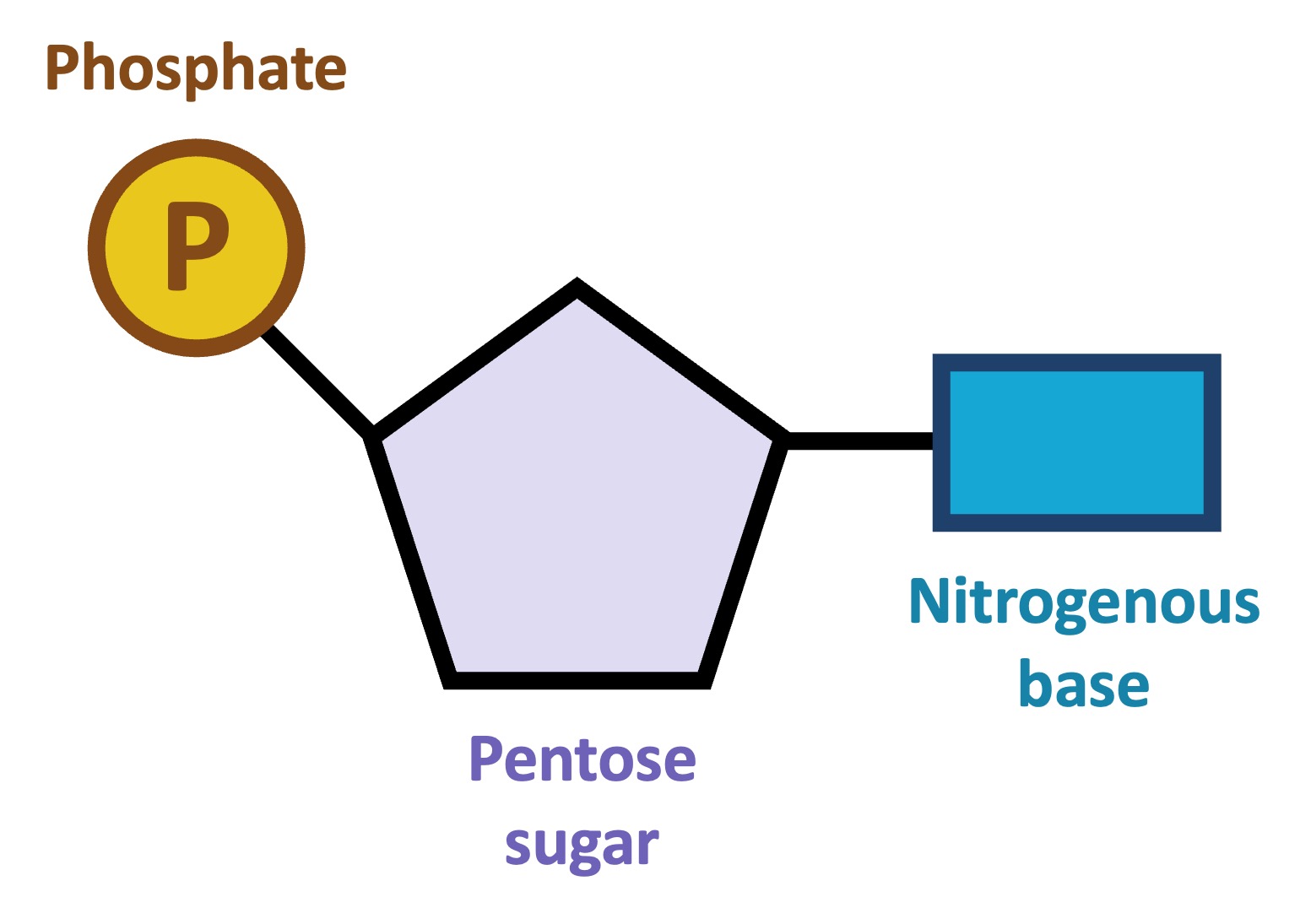
Nucleotide
The building block of DNA, which is made up of phosphate, a deoxyribose sugar, and a nitrogenous base. Connect to others through weak hydrogen bonds formed between bases
Chromosome
A large length of DNA tightly wrapped around proteins, which contains many genes. Usually has 23 different versions in a human body. Extremely information dense in order to make being pulled apart for cell division easier. The amount of these in each organism vary (e.g., 46 in humans)
Genes
A large section of DNA which codes a specific protein or trait, such as skin type or dimples
DNA vs Chromosomes vs Genes
DNA is substance which includes information on how the body should work and be built. Chromosomes are made up of long strands of tightly wound DNA around histones, which are incredibly information dense and found in the nucleus. Genes are sections of DNA which code a specific thing, and make up chromosomes
Cell Division
The process by which a single cell splits into daughter cells. Comes in two forms, mitosis and meiosis. Is important as it provides an exact replica of DNA for a cell in the body. Works due to the complementary base pairing rule, meaning each half of a chromosome be a template for another half
Process of DNA Replication
DNA molecule unwinds from chromosome
DNA molecule unzips (gets cut in half)
Two separate strands form
Each side of the DNA acts as a template for new strands using spare nucleotides in the cell
Each new side zips up (is put back together)
Identical copy of DNA is created
Homologous Chromosome
Two chromosomes inherited from each parent that have the same purpose (same genes coding same things), and are similar in height, length and centromere location, though having different alleles. Are paired together in a karyotype, and are only not found between and X and Y chromosome
Mitosis
The process by which a cell splits it’s chromosomes in half to create double the amount of chromosomes, in order to create two daughter cells (would be diploid cells represented by 2n). Used for growth, repair and replacing
Meiosis
The process by which a cell splits it’s chromosomes in half to create double the chromosomes, which are then split between 2 daughter cells, then into 4 daughter cells (meaning each has half the amount of chromosomes). These cells are known as gametes, represented as haploid/n, and are for sexual reproduction
Fertilisation
The process in which two gametes with half the number of chromosomes combine to create one cell with the total amount of chromosomes
Why Meiosis has Two Divisions
As one division individually splits each chromosome in half, in order to create double the amount of total chromosomes. The second puts half of the total group in one cell, and the other half in another cell
How Genetic Information comes from both Parents
Both parents’ gametes (egg and sperm) are gametes and combine to create a person’s set of 46 chromosomes
Karyotypes
A visual display of all of a person’s chromosomes, with the autosomes arranged from longest to shortest in their homologous pairs. Sex chromosomes are found at the end, generally with male karyotypes having XY chromosomes, and female having XX
Amounts of Chromosomes in Other Animals
Goldfish - 25 chromosomes pairs
Fruit fly - 4 chromosome pairs
Cat - 19 chromosome pairs
Inheritance
The process by which genetic information is passed down from parents to children
Contribution of Gregor Mendel to Inheritance
Created three key principles of inheritance, being:
Inheritance is determines by genes passed onto offspring
Offspring inherit one gene for each trait
A trait may not show up in one offspring, but can still appear in the next generation (recessive genes)
Allele
Alternative forms of the same gene (e.g., hair colour having brown and blonde)
Genotype
The genetic makeup of a specific trait (e.g., blue eyes as Bb). Is communicated as two letters, with capital letters being first and representing the dominant trait, and lowercase being recessive traits
Phenotype
The physical expression of genes on the body, and can be influenced by external factors. For example, being short (even if having tall genes due to poor nutrition)
Genotype vs Phenotype
Genotypes refer to the actual combination of alleles inherited in an organism from it’s parents. Phenotypes differ in that they are the physical expression of alleles, so what the body looks like, and while influenced by genotypes, can also be affected by external factors, like nutrition, environment, etc.
Homozygous
Also known as pure-bred, where a trait is made up of two of the same alleles. Is found in two forms, dominant (having both dominant traits, e.g., BB) and recessive (having both recessive traits, e.g., bb)
Heterozygous
Also known as hybrid, where a trait is made up of a dominant and a recessive allele, e.g., Bb. Will always be displayed as the dominant trait
Punnet Square
Visualisation of the different possibilities of the passing on of genes. If sexes are known, the male alleles (letters) will be put down the column, while the female alleles will be put across the row
X Linked Traits
Traits which are present on the X Chromosome. These traits are more commonly passed onto men as generally they are recessive, and generally men have only one X Chromosome (meaning there can be no dominant gene to overcome the recessive). On the other hand, women generally have two X chromosomes meaning there are more possibilities for a dominant trait to be passed on to overcome the recessive
Autosomal vs Sex Linked Inheritance
Autosomal refers to any chromosomes besides the X and Y chromosomes, while sex-linked refers to only the X and Y
Sex Linked Inheritance Notation
X Chromosome is always put before any other chromosomes, and X Chromosomes will have the trait letter in superscript (e.g., XᴬXᵃ). Y will usually have no trait unless it is relevant
Periodic Law
The law created by Dmitri Mendeleev, in which he states that by arranging elements in order of their atomic number, patterns in their properties are formed. Through this, he arranged the periodic table in groups and periods to show the patterns (like valence electrons, and outer shells)
The groups (vertical) show the elements with the same number of valence electrons, while the periods show the elements with the same number of outer shells
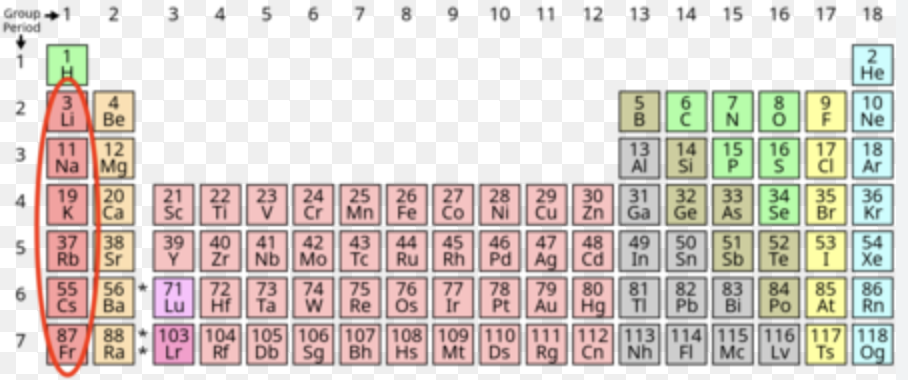
Alkali metals
Most of Group 1, and is lithium , sodium, potassium, rubidium, caesium, and francium. One of the most reactive groups, forming a cation, and has a charge of 1+ when stable. Are soft, low density, low in melting/boiling points, and become more reactive as you go down, as the more outer shells an atom has, the less attraction is has toward the valence electrons (as they are far away), allowing it to lose the electrons easier
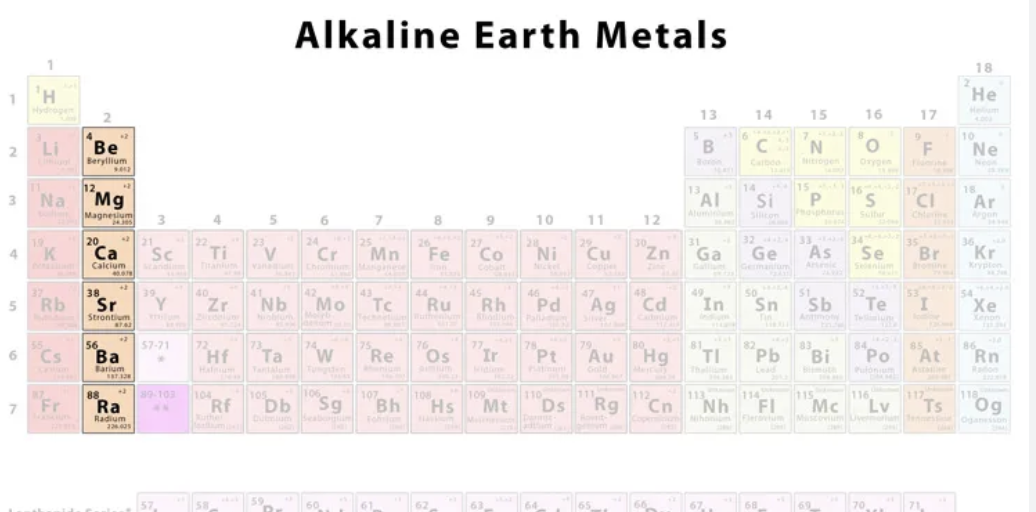
Alkaline Earth Metals
Is Group 2, being beryllium, magnesium, calcium, strontium, barium, and radium. One of the second most reactive groups, forming cations with a charge of 2+. Are soft, low density, low in melting/boiling points, and become more reactive as you go down, as the more outer shells an atom has, the less attraction is has toward the valence electrons (as they are far away), allowing it to lose the electrons easier
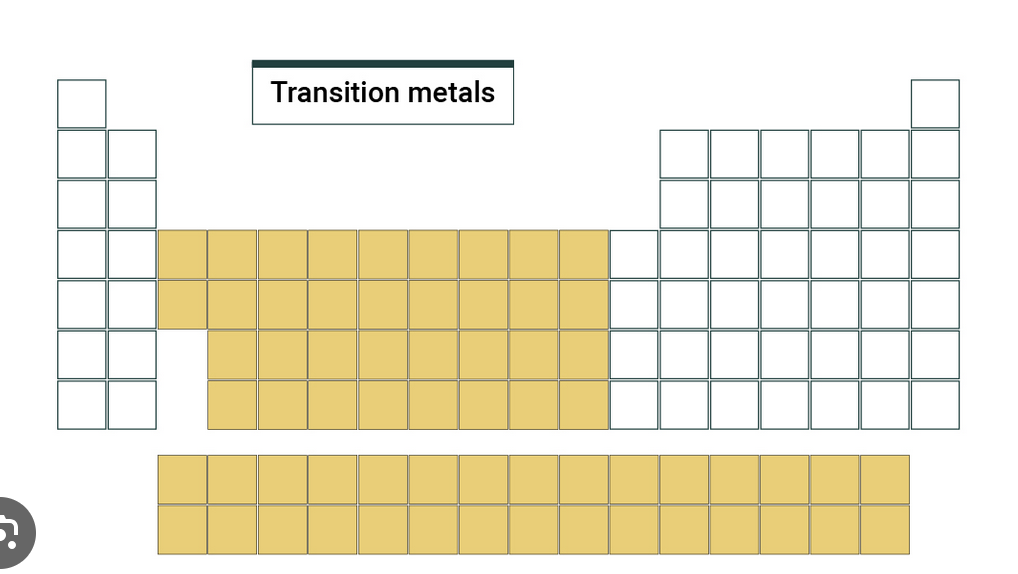
Transition Metals
The elements between group 3 to 12, being able to form cations, and are fairly unreactive when compared to alkali metals and halogen, though can react. Are strong and hard, high density, and have high melting/boiling points
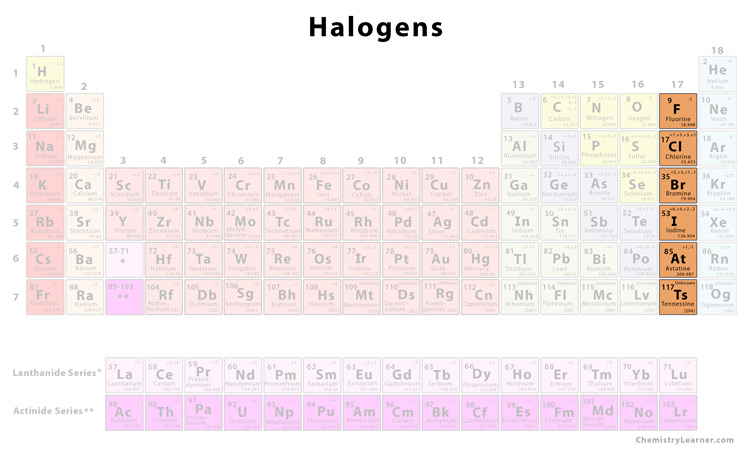
Halogens
Halogens
The elements in the 17th group of the periodic table, including Fluorine, Chlorine, Bromine, Iodine, Astatine, and Tennessine. Form anions with a charge of 1-. Are brittle when solid, have low melting/boiling points, and become less reactive as you go down the group, due to having less attraction to pull in electrons as there are more outer shells in the way
Noble Gases
The elements of Group 18 which are completely unreactive due to having a full valence shell.
Metals
The elements that include the alkali metals, alkaline earth metals, transition metals, and post-transition metals, which form cations, and bond together to create metallic bonds
Non-Metals
Elements that include the halogens, noble gases, and other non-metals (carbon, nitrogen, hydrogen, oxygen, phosphorous, sulfur, selenium), which if in ions, form anions, and when bonded together, create covalent bonds
Metalloids
The group of Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium which share properties with metals and non-metals, such as being as able to be anions or cations
Electron Shell Filling Order
2, 8, 8, 2, though this ends at Calcium and changes to 2, 8, 18
Valence Electron Shell
Outermost electron shell, which either seeks to be removed (Cations), to be filled (anions), or nothing (noble gases)
Charges of Groups when Ions
Group 1: 1+
Group 2: 2+
Group 13: 3+
Group 15: 3-
Group 16: 2-
Group 17: 1-
Process of Ion Formation
When a metal and a non-metal come close to each other, if they have incomplete valence shells, the metal may give it’s valence electron to the other element. The metal, as it loses electrons, will gain a positive charge and become a cation, while the the non-metal will gain a negative charge and become an anion. If both of the elements have full valence shells, they become stable
Neutral Atom vs Charged Ion
Neutral refers to an atom without any charge, meaning it has an equal amount of protons to electrons, while charged ions have more or less electrons than protons
Bond Naming Process
Ionic bonds always have the metals first, and then the non-metals second. The name will have the metals, and then the non metals with the amount of the nonmetal + ide/ine/etc. (will be on valency chart). Metallic bonds can only be made of one element, so it is just that, and covalent bonds have the first as its element, and only having its number if there is more than one of it (e.g., dihydrogen monoxide in H2O)
Ionic Bonding
A form of bonding between two or more metal and non metal ions, in which the metals transfer their valence electrons to the non-metals, allowing both to become stable
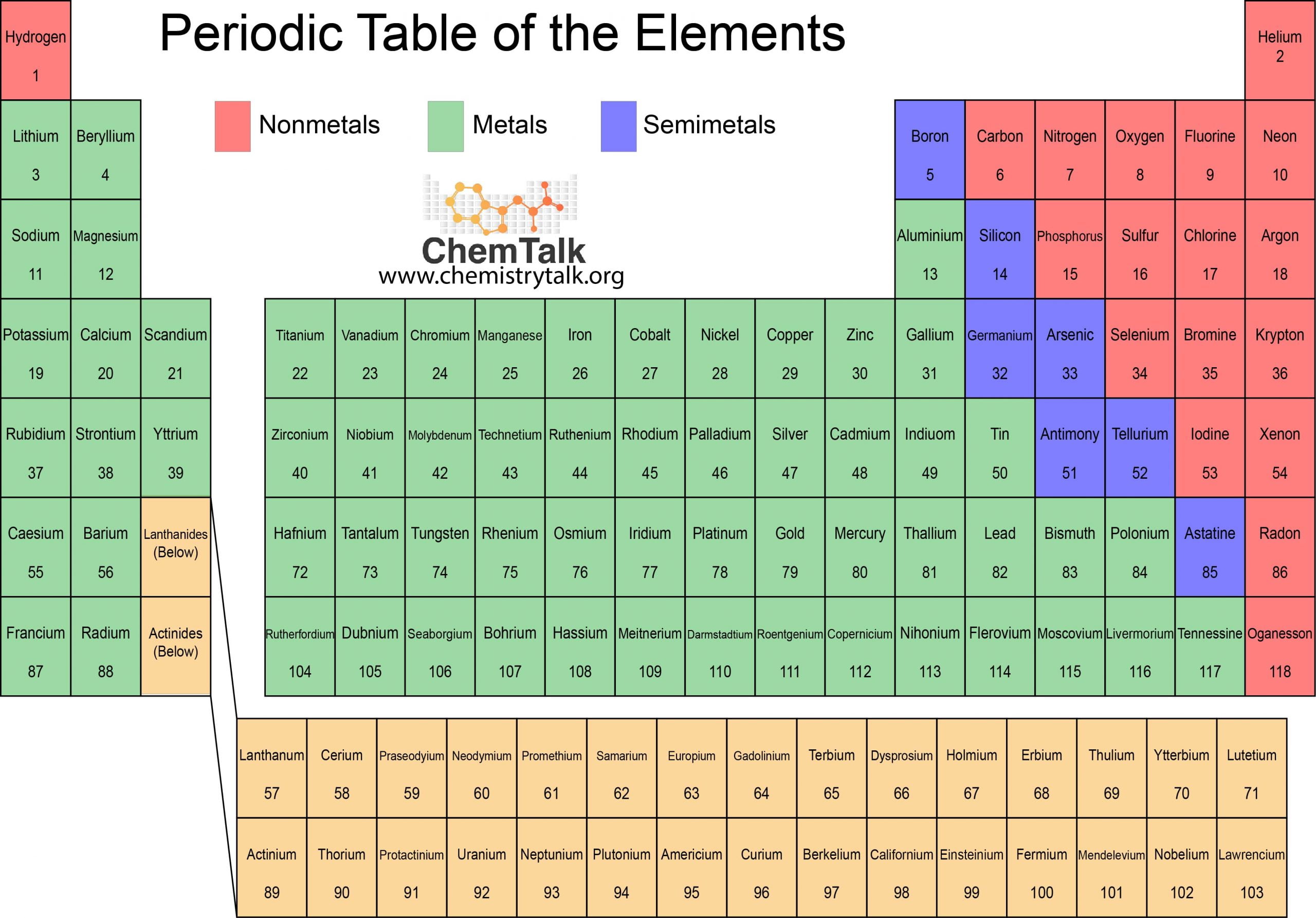
The Lattice in Ionic Bonding
The structure of ionic bonding, which is made up of alternating positive and negative ions that are equally spaced apart, and have a strong electrostatic attraction keeping them in place
Properties of Ionic Bonding
Non-malleable due to movement the positive ions will put them closer other positive ions, and cause them to repel (same as with negative ions)
Non-conductive when solid as they are not moveable due to their strong electrostatic attraction
Conductive when liquid as they can move, and are charged particles
Have high melting/boiling points due to having strong electrostatic attractions (meaning much energy is required to break bonds)
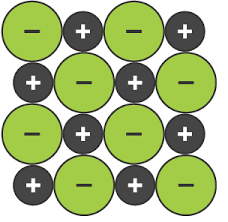
Metallic Bonding
A form of bonding between two or more of the same metal, in which the metals remove their extra valence electrons and form a layer of positive metal ions, connected by a sea of delocalised electrons, giving it a high electrostatic attraction keeping it in place
Metallic Bonding Properties
Malleable due to the sea of delocalised electrons still connecting metal ions when they move as the sea can move
Conductive due to the sea of delocalised electrons being able to carry charges
High melting and boiling points due to the strong attraction from the electrons keeping the positive metal ions together
Covalent Bonding
A form of bonding between two or more non-metals share one or more electrons with each other to allow both to reach stability
Properties of Covalent Bonding
Malleable as there is only little attraction (Van Der Wall’s charges) holding multiple molecules together, only having attraction to keep one molecule intact
Non-conductive as the molecule has a neutral charge due to there being no transfer of electrons, only sharing
Low melting/boiling points for the same reason as why they are malleable (there being little attraction connecting each molecule)
Collision Theory
The theory that for a chemical reaction to occur:
Particles must collide
with enough energy to break their chemical bonds
in the correct orientation to make new bondse
Method of Changing the Rate of Reaction
Concentration/Pressure
Agitation (stirring)
Surface Area
Temperature
Catalystst
Concentration/Pressure
A method of changing the rate of reaction, in which the concentration of the reactants affects how fast it occurs. This occurs as by increasing the concentration, you increase the amount of reactants in the space. This means they are more likely to collide, and thus more likely to collide in the correct orientation.
This is also includes the pressure of the substance for gases, as the more compressed gases are, the less space there is for the reactants to move around and thus will collide faster.
This affects the “particles must collide” aspect of collision theory
Temperature
A method of improving the rate of reaction, in which the temperature of the reactants affect how quickly the reaction can occur. This happens as by increasing their temperature, you increase the amount of energy they have, which:
Allows them to move faster and thus collide quicker
Allows for more particles to have sufficient energy to react with other reactants
This affects the “particles must collide” and “with sufficient energy” parts of collision theory
How the Rate of Reaction changes throughout a reaction
Over time, the rate of reaction decreases as less reactants become available due to how they have become products, resulting in the reaction slowing down as the reactants will collide less frequently, and thus collide in the correct orientation slower
Examples of Catalysts
Enzymes, biological catalysts found in the body that put catalysts in the correct orientation, used in things like respiration
Catalytic converters, which are parts of cars that use catalysts to react with the harmful exhaust gases and convert them into harmless exhaust gases
Enzymes, which are also used in washing powder to help clean clothes
Scalar Quantities
A quantity which only measures magnitude/amount, like grams being the amount of mass, and metres being amount of distance
Vector Quantities
A quantity which measures both magnitude, and direction, like displacement being amount of distance and the direction from starting point
Vector Diagram
A grid which is used to map the movements of an object. Used for questions in which you are told movements, (e.g., the car moved 3km north and 4km east). Graphed by drawing out the movements on the grid according to the key. (e.g., 3km north = 3 up, 4km east = 4 right).
Distance is found by adding all movements together. Displacement is found by using pythagoras to find the distance between the starting point and the ending point. (e.g., distance = 3 + 4 = 7km. displacement = √(3²+4²) = 5km)
Speed/Velocity Equation
v = s ÷ t, where:
v = velocity or speed
s = displacement or distance
t = time
How to find Velocity, Distance, Displacement, and avg. Speed in a Displacement Time Graph
Velocity: Using rise/run
Distance: Add up lengths of the line itself
Displacement: Final point displacement - First point displacement
Avg. Speed: Distance/time, using the distance moved in the section and finding time by doing (time value at the end of the section) - (time value at the start of section)
Displacement Time Graph
A graph which shows the changes in displacement of an object over time. A flat area means an object is still, a displacement of 0 means that the object returned to the original point, and a negative displacement means the object is behind the point where they started
Velocity-Time Graph
A graph that shows the velocity of an object over time. A flat point shows an object moving at a constant speed, and a point of 0 velocity means an object is stationery. If velocity goes below 0, an object is moving backwards
How to find Acceleration and Displacement on a Velocity Time Graph
Acceleration: rise/run
Displacement: Calculating the area of the shape under the line
Acceleration Formula
a = (v-u)/t, where:
a=acceleration [m/s/s]
v=final velocity [m/s]
u=initial velocity [m/s]
t=time [s’
Acceleration
Uses the symbol a, and is the rate of change of speed over time, using the unit ms-2 -(or m/s/s). Can be a negative if something slows down
How to convert between km/h and m/s
If going from km/h TO m/s, then divide by 3.6
If going from m/s TO km/h, then multiply by 3.6
Force
Any push, pull, or twist, which causes an object to change in some way, and is measured in Newtons (N)
Can make it start/stop moving, speed up, slow down, change direction, or change shape
Newton’s First Law
An object which is in motion will remain moving until opposed, and an object at rest will stay at rest until moved. Can be seen in examples like a magician pulling a tablecloth, or a ball on a skateboard
Also known as the law of inertia
Newton’s Second Law
Force is equal to mass times acceleration, meaning that more accelerations is more force, and more mass requires more force to accelerate
Can be simplified as F=ma, where F=net force [N], m=mass [kg], and a=acceleration [m/s/s]
Weight
A force, which can be measured in Newtons.
Can be found in the equation Fg=mg, where Fg is gravity [N], m is mass [kg], and g = acceleration due to gravity [on earth is 9.8m/s]
Newton’s Third Law
For every action, there is an equal opposite reaction
Means that each force applied causes another force to react to it (referred to as applied forces and reaction forces)
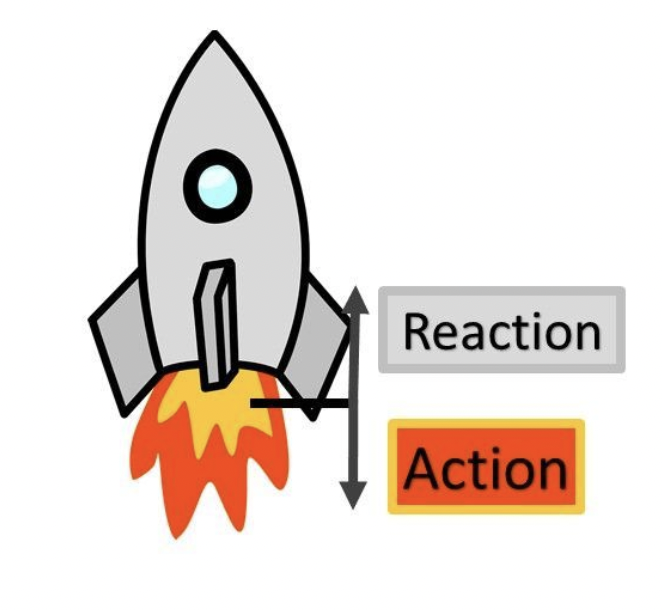
Newton’s Third Law Examples
When a tennis racquet hits a ball, the net of the racquet tries to spring the ball back
When anything with weight is on the ground, the ground pushes against it with normal force
When a gun is shot, the acceleration of the bullet pushes the gun back
Biodiversity
The variety and variability of species on Earth, which is a function of evolution
Genetic vs Species vs Ecosystem Diversity
Genetic diversity is the diversity of genes in one species, while species diversity is the diversity of species in one ecosystem, while ecosystem diversity is the diversity of ecosystems in one location
Evolution
The process of which mechanisms drive a species to change over time, which can take millions of years
Importance of Variation in Evolution
In terms of Genetic Diversity, allows for some members of a population to survive changes to the environment or diseases, which otherwise would affect the entire population. This resistance to the change/disease some members of the species have is then able to be spread to the rest of the species
In terms of species diversity, allows ecosystems to survive changing populations of species, like if one prey organism goes extinct, predators still have access to other prey. Allows for the different species of the ecosystem to evolve and adapt to different conditions
Methods that lead to Variation
Independent Assortment - the varying ratios of maternal and paternal chromosomes in a diploid cell (may have more maternal, more paternal, or equal)
Crossing Over/Recombination - when chromosomes come in contact and swap pieces
Mutations - a change in the DNA sequence
Effect of Environment on the Phenotype
The environment of an organism has a great affect on how an organism looks. If an organism has poor nutrition, is exposed to too much sunlight, etc., it’s genes/alleles will not change (meaning there is NO increase of variation), but it will look different.
Basically, the environment can cause a change in the phenotype, but not a change in the alleles/genotype
Population
The amount of individuals in a species in a location at a specific time and place
Gene pool
The combination of all genes and alleles present in a population
Allele Frequency
The rate that organisms in a species get alleles of a gene. For example, the rate a frog gets green skin colour may be 75%, while the rate it gets blue skin colour may be 25%
Relationship between Allele Frequency and Gene Pool
Both are methods of describing the alleles present in a species. The gene pool depicts all of the alleles in a population, while the allele frequency depicts how often these alleles show up
The Mechanisms of Evolution
Mutation
Natural Selection
Gene Flow
Genetic Drift
Mutation
A change in the DNA sequence, and a key function of evolution as it is the only way to increase genetic diversity. Can increase variation if change occurs in a gamete, though usually doesn’t affect genotype or phenotype (but can)
Natural Selection
A mechanism of evolution in which organisms in a species are introduced to selection pressures, which cause only some members to be able to survive/reproduce. Those with the best genes suited to these selection pressures survive and reproduce more often, passing on the more effective genes (basically survival of the fittest)
Gene Flow
A mechanism of evolution where an organism/s from one population enters another population, spreading it’s genes/alleles. For example, green bug entering a population of yellow bugs, eventually making half the bugs green
Genetic Drift
A mechanism of evolution where the gene pool randomly changes, causing a species to evolve. This is usually because of a random event that kills many members of a species, only allowing the random survivors to spread their genes and make a new population. For example, an earthquake killing most birds with red stripes, may make birds with black stripes more common
Selection Pressures
Things (can be living or non-living) that stop the organisms of a species from being able to survive/reproduce. These drive natural selection. For example, pollution, predators, disease, temperature, etc.
How Gene Pools Change over time Due to Natural Selection
Selection pressures can greatly affect a species, potentially leading them to extinction. After a while, the species will begin developing traits more resistant to these pressures, meaning the gene pool becomes filled with mainly these better alleles.
For example, the peppered moth in the 1850s was white as it hid on white trees, though sometimes were born black. When trees turned black due to pollution in the 1900s, the black body allele became more common in the gene pool.
For example, when a human uses antibiotics, the meds kill most but not all of the bacteria. The surviving bacteria quickly breeds and recreates a colony, filled with more bacteria resistant to the antibiotics. The gene pool becomes filled with a resistance to antibiotics
Process of the formation of a new species
Variation - where a species is very genetically diverse
Natural Selection/Genetic Drift - where selection pressures or a random event causes only those with the traits to survive to pass on their genes
(both change allele frequency)
Evolution accumulates over time - slowly, the species becomes more and more different than the original population
New species is formed - the new population can now now longer mate and make fertile offspring
Artificial Selection
The process by which humans selectively breed species to give them desired characteristics, meaning people are the selection pressure. This can be seen in domesticated pigs, who have been bred to heel more meat, and modern bananas, which have been bred to minimise/remove their seeds
Natural Selection vs Artificial Selection
Natural selection goes over thousands of years and is driven by certain selection pressures that force a species to develop better traits to survive. Artificial selection goes over a couple generations and is driven by humans breeding for specific characteristics in a species
Species
Groups of organisms that are able to produce fertile offspring together
Speciation
The process by which new species are created
The Forms of Evidence for Evolution
Fossils
Biogeography
Comparing Anatomy
Comparing DNA and Proteins
Fossil
A preserved trace of an organism that lived long ago, and can been seen in things like skeletons, footprints, impressions, etc.
Process for Fossilisation to Occur
Organism is buried in sand/mud/dirt (prevents any damage or disruption)
Organism is further buried by water/dirt/mud to put it under higher pressure (allowing minerals to enter hard parts of body and turn it into stone)
Soft parts are not generally not preserved and are lost (but can leave imprints)
Organism is found later in sedimentary rock
How Fossils Support the Theory of Evolution
The age of fossils can be found through processes like carbon dating, which allow scientists to prove that some species existed in certain areas and changed over time. Through this, scientists are able to prove that the fossils were the ancestors of other organisms