ap bio unit 2 review
Cell Organelles, Membranes, and Transport
Cell Organelles and Their Functions
two major types of cells:
- prokaryotic: simpler in structure; found in bacterial organisms
- eukaryotic: contain membrane-bound organelles; more complex; found in animals, plants, fungi, and protists
- all cells (prokaryotic and eukaryotic) have the following: genetic material, ribosomes, cytosol, and a plasma membrane
- the genetic material in prokaryotes is circular and stored in the center of the cell called the nucleoid region
- plasmids: small circular pieces of genetic material stored outside of the chromosome; often found in some forms of bacteria
- genetic material in eukaryotes is linear and stored in a membrane-bound nucleus
ribosomes: functions in protein synthesis; found in prokaryotic and eukaryotic cells; made of proteins and ribosomal RNA (rRNA)
- sizes of the large and small subunits of ribosomes vary in eukaryotic and prokaryotic cells
- during translation: ribosomes assemble amino acids into polypeptide chains according to the mRNA sequence
- there are free ribosomes in the cytosol and organelle-bound ribosomes on the membrane of the rough endoplasmic reticulum
endoplasmic reticulum: formed of two parts (smooth ER and rough ER)
- rough ER: covered with ribosomes; functions in proteins synthesis
- smooth ER: does not contain ribosomes; functions in lipid synthesis and detoxification of harmful substances in the cell
golgi complex (golgi body/apparatus): a stack of flattened membrane sacs (cisternae); functions in controlling the modification and packaging of proteins for transport
- lumen: interior of cisternae; contains necessary enzymes for the golgi complex to function
- proteins made on the free ribosomes of the rough ER are sent to the golgi body to be modified and packed into vesicles for transport throughout the cell
- vesicles: structure within or outside a cell, consisting of liquid or cytoplasm enclosed by a lipid bilayer
lysosomes: membrane-bound sacs containing hydrolytic enzymes that are used in various functions including digestion of macromolecules, breaking down of worn-out cellular parts, apoptosis, or destroying bacteria in the cell
- hydrolytic enzymes: break down protein, lipids, nucleic acids, carbohydrate, and fat molecules into their simplest units
vacuoles: membrane-bound sac that functions in the storage of food or water for the cell, water regulation, or waste storage (until it can be eliminated)
- plant vacuole: large central vacuole that helps regulate the water balance of cell
- well-hydrated plant cells will have proper turgor pressure, which is maintained by the vacuole in the center of the plant cell
- turgor pressure: provides structural integrity to each cell and to the tissue as a whole; pushes the plasma membrane against the cell wall and causes in-plane mechanical tension within the cell wall
- animal vacuole: generally small and help sequester waste products
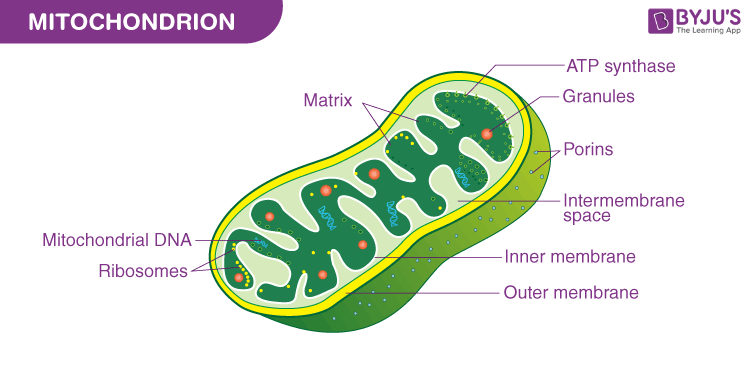
mitochondria: produces energy (ATP) for the cell; contains a double membrane (smooth outer membrane and folded inner membrane)
- the folded inner membrane allows for increased surface area, which increases the efficiency of ATP production during cellular respiration
- the double membrane allows for mitochondria to form proton (H+) gradients which are necessary for ATP production
- matrix: center of the mitochondria; fluid containing enzymes; the location where the krebs cycle (citric acid cycle) occurs
- mitochondria also contain their own ribosomes and mitochondrial DNA (mtDNA)\
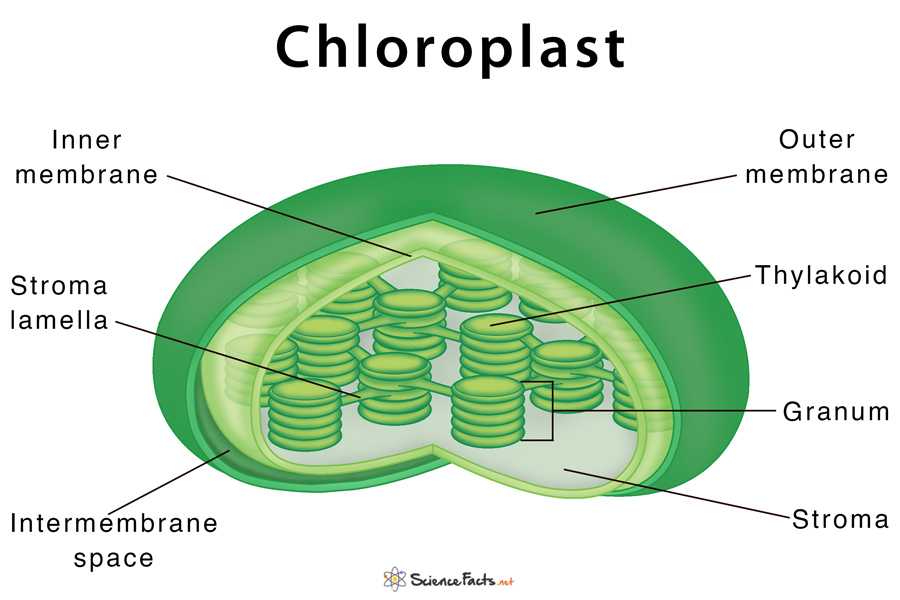
chloroplasts: found in plants and algae; carry out photosynthesis; double membrane organelle with smooth outer membrane and structures inside
- thylakoids: pancake shaped membraneous sacs stacked into structures; functions in light-dependent reaction
- grana: the structures thylakoids are stacked into
- stroma: liquid in chloraplast surrounding the grana; enzymes in stroma function in light-independent reactions
- contain their own dna (cpDNA)
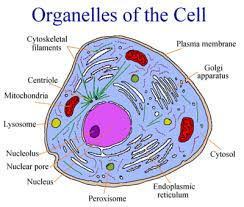
centrosome: found in animal cells; helps microtubules assemble into spindle fibers (used in cell division)
- defects in centrosome cause dysregulation of cell cycle (and causes some cancer)
amyloplasts: starch molecule that store excess glucose produced during photosynthesis; commonly found in starchy root vegetables (ex. potatoes)
several structures are found in plant and animal cells:
- peroxisome: helps oxidize molecules and break down toxins in cells
- nucleolus: not membrane bound organelle; region in the nucleus where ribosomes are assembled
- cytoskeleton: fibers that help give cells their shape and move items in cell
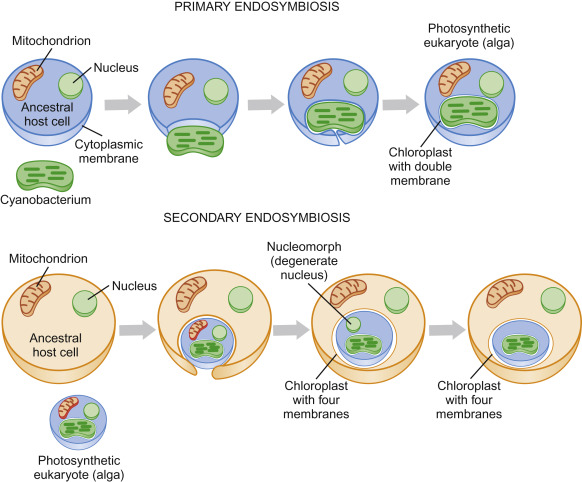
Endosymbiosis Hypothesis
endosymbiosis hypothesis: states that membrane-bound organelles (mitochondria and chloroplasts) were once free-living prokaryotes that were absorbed by larger prokaryotes
- the prokaryotes became interdependent of each other and the larger prokaryotes evolved into membrane bound organelles
reasons for this theory:
- mitochondria and chloroplasts have their own DNA (circular like prokaryotic DNA)
- mitochondria and chloroplasts have their own ribosomes (similar in structure to prokaryotic ribosomes)
- mitochondria and chloroplasts are produced by binary fission (similar to how bacteria reproduce)
Advantages of Compartmentalization
- membrane-bound organelles form compartments to increase their efficiency
- compartmentalization: allows cells to separate enzymes involved in different metabolic processes
- this reduces the risk of cross-reacting, which would decrease efficiency of the cellular processes
The Importance of Surface Area to Volume Ratios
- many eukaryotic organelles (ex. mitochondria) have folds in their membranes to increase surface area
- prokaryotes can fold their single membranes to also increase surface area
- the larger the SA:V ratio, the more efficient the cell is
- as radius increases, the ratio decreases
- larger cells have a lower SA:V ratio, making them less efficient in certain functions
Structure of Plasma Membranes
plasma membranes are selectively permeable (some materials can cross and others cannot)
- selective permeability allows the cell to maintain its internal environment
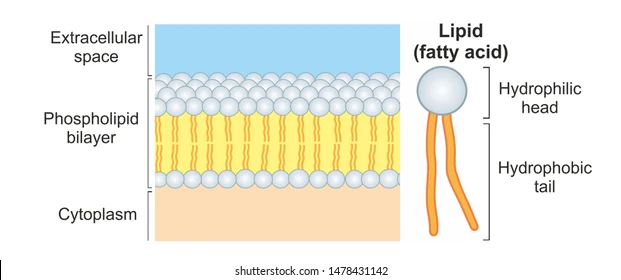
plasma membranes are made of a phospholipid bilayer
- phospholipids have a hydrophilic phosphate head and two hydrophobic tails
- tails orient themselves away from internal (aqueous) environment
- phospholipid bilayer contains glycoproteins, glycolipids, and steroids
- these molecules can move throughout the bilayer and allow the cell to adapt adn respond to changing environmental conditions
- proteins (in membrane): used to transport materials, participate in cell signaling processes, anchor the cell in place, and catalyze chemical reactions
- glycoproteins and glycolipids: used in cell recognition
- steroids: adjust membrane fluidity in response to changing environmental conditions and needs of the cell
- fluidity of molecules in plasma membrane gives it the term “fluid mosaic model”
Crossing (and Not Crossing) Plasma Membrane
- phospholipid bilayer makes cell membrane selectively permeable
- small hydrophobic molecules (ex. oxygen, carbon dioxide, and nitrogen) can move between phospholipids and in/out of the cell
- larger polar molecules and ions cannot pass through as easily without help
- large polar and charged molecules must use membrane channels or transport proteins to enter/exit cell
- small polar molecules (ex. H2O) can pass in small quantities; larger amounts also must be assisted
- aquaporins: special proteins that allow for the movement of (most) water in/out of cells
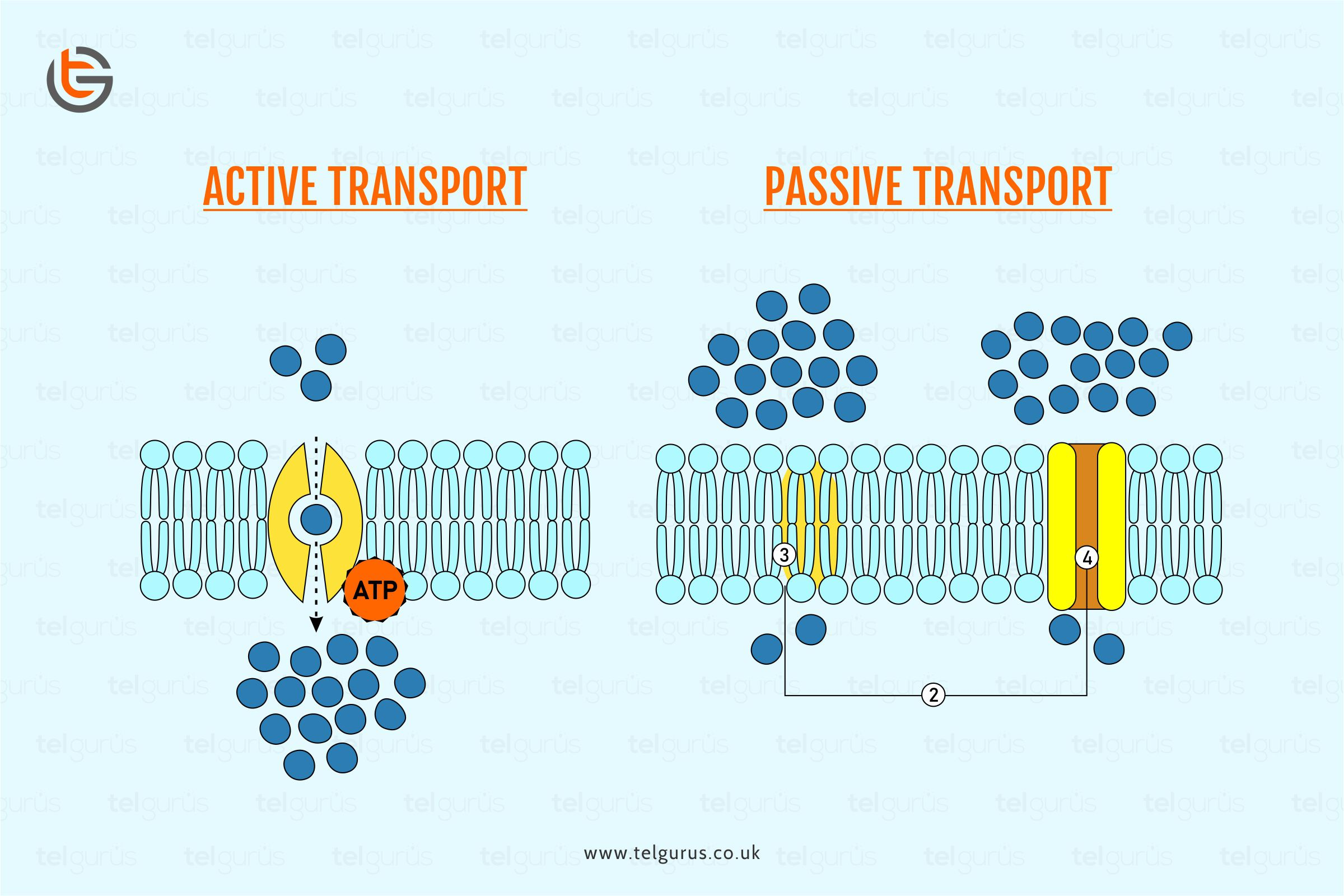
Passive Transport
- passive transport: movement of molecules in/out of cell without energy required; molecules move from areas of high concentration to areas of low concentration (moving “down” concentration gradient)
- diffusion: movement of molecules down concentration gradient without energy required
- osmosis: diffusion of water molecules down a gradient and across a membrane
- facilitated diffusion: process of passive transport with use of membrane protein; used for polar/charged molecules
- aquaporins are an example of membrane proteins (only used for water)
- channel proteins: can allow the passive transport of ions (ex. Ca+2 or Cl-1) down the concentration gradient
- rate of facilitated diffusion is limited by the number of membrane proteins available
Active Transport
active transport: movement of molecules from areas of low concentration to high concentration; movement of molecules “against” concentration gradient requires the input of energy
Na+/K+ pump: prime example of active transport
- membrane protein requires the input of ATP to pump Na+ ions from lower concentration to higher concentration outside the cell
- membrane protein pumps K+ ions from areas of lower concentration to higher concentration inside the cell
- for every 3 Na+ ions pumped outside cell, 2 K+ ions are pumped into cell
- results in higher concentration of positive ions outside of cell and helps cell maintain membrane potential
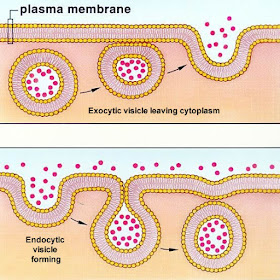
endocytosis and exocytosis are also forms of active transport (both require input of energy)
- endocytosis: used by cell to take in water and macromolecules with vesicles formed from plasma membrane
- exocytosis: vesicles (with molecules) are merged with cell membrane and molecules in vesicles are expelled from cell
Movement of Water in Cells
Water Potential
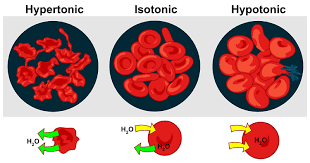
hypotonic: lower concentration of solute outside than inside cell; higher water potential
- cell swells and bursts
hypertonic: higher concentration of solute outside than inside cell; lower water potential
- cell shrinks and shrivels
isotonic: equal concentration of solute inside and outside cell
- cell pressure is maintained
water potential: the potential energy of water in a solution; the ability of water to do work
- the more water there is in a solution, the higher the water potential
- water flows down concentration gradients (higher concentration to lower concentration)
Calculating Water Potential
- solute potential (Ψs): water potential due to solute concentration
- Ψs depends on how many particles in the solute form the solution and the temp. of the solution
- Ψs = -iCRT
- i: ionization constant; function of how many particles or ions will form the solution in a given solute
- covalent compounds: i = 1 (ions don’t separate)
- ionic compounds: i depends on how many ions form in the solution (ex. NaCl forms 2 ions (Na+ and Cl-) so i = 2)
- C: concentration of solute in solution; as concentration increases, solute potential decreases
- solutes with more solute (higher solute concentration) will have lower water potential (if all other variables are equal)
- R: pressure constant; R = 0.0831 L-bars/mol-K
- T: temperature of solution; only in Kelvin
- pressure potential (Ψp): water potential due to pressure on system
- most biological systems are open to equilibrium in their environments which eliminates pressure in the equation and it becomes: Ψ = Ψs
- when solution is open to atmosphere, Ψp is zero
Osmolarity and Regulation
- osmolarity: total concentration of solutes in solution
- living organisms need to closely regulate internal solute concentration and water potential (to far away from proper conditions could lead to death)
- contractile vacuole: specialized organelle used to store excess water until it is pumped out of the cell; allows cells to maintain internal solute concentration