BJU 6th edition Physical Science chapter 10
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
45 Terms
acids
substances that produce hydrogen ions in a solution with water

hydrogen ion=_____________
proton
aqueous solution
water-based solvent
hydronium ions
In water, the hydrogen ions combine with water molecules to form ________________________.
the more hydronium ions in a substance the more _____ the substance is.
acidic
Acids can...
chemically react with metals in a single-replacement reaction
T/F: Pure water conducts electricity
False
1 multiple choice option
T/F: Ions dissolved in water conduct electricity
true
1 multiple choice option
indicator
a substance that will change color in the presence of an acid or a base
litmus
a kind of dye
Characteristics of a base
-proton acceptors
-slippery feel
-conductivity
-color change with indicators
-bitter taste
bases are substances that produce
Hydroxide ions (OH-) in an aqueous solution
hydroxide ions can
accept the positive ions of an acid to make water
Bases feel
slippery like soap
Bases turn
red litmus paper blue
1 multiple choice option
A bitter taste indicates the presence of a ______
base
1 multiple choice option
Examples of a bitter taste
caffeine, unsweetened chocolate

What determines the strength of an acid?
how easily it loses hydrogen ions
If all or most of the molecules in an acid will ionize in water, then the acid will produce many hydrogen ions in solution and be called a ____________.
strong acid
most of the molecules in __________ do not ionize in water, so fewer hydrogen ions are produced
weak acids
the more ions an acid produces in solution, the more________ it is
reactive
strong bases
include those that readily dissociate, producing large numbers of hydroxide ions
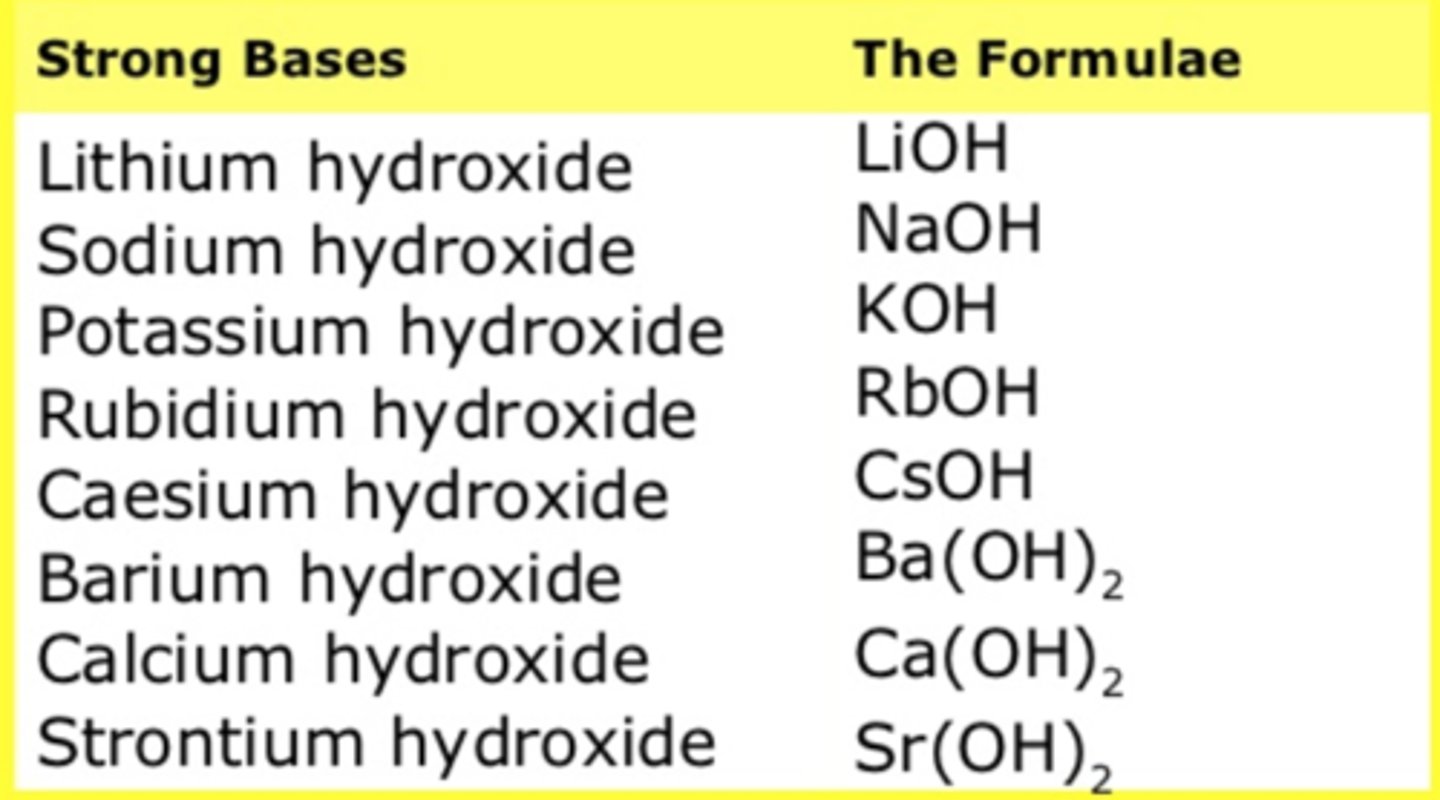
common in base's formulas
OH
Weak bases
produce hydroxide ions by ionizing in a reaction with water
concentration
the amount of solute per unit of solution
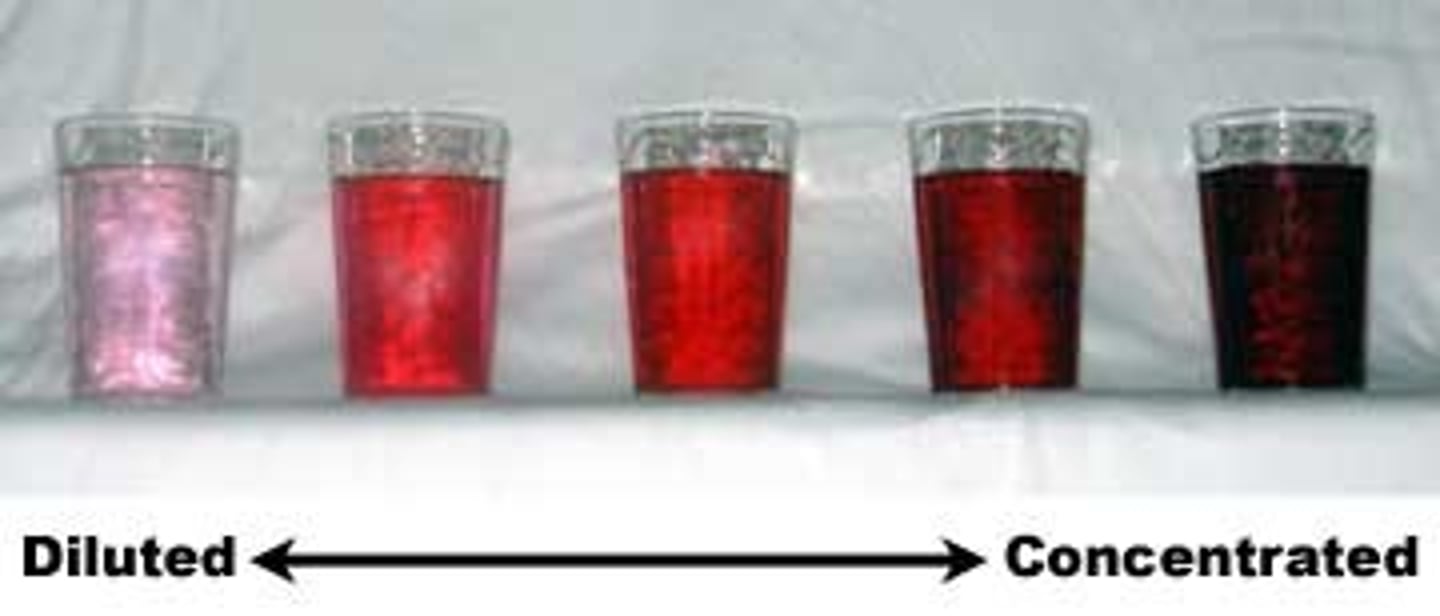
concentration is measured
on a pH scale
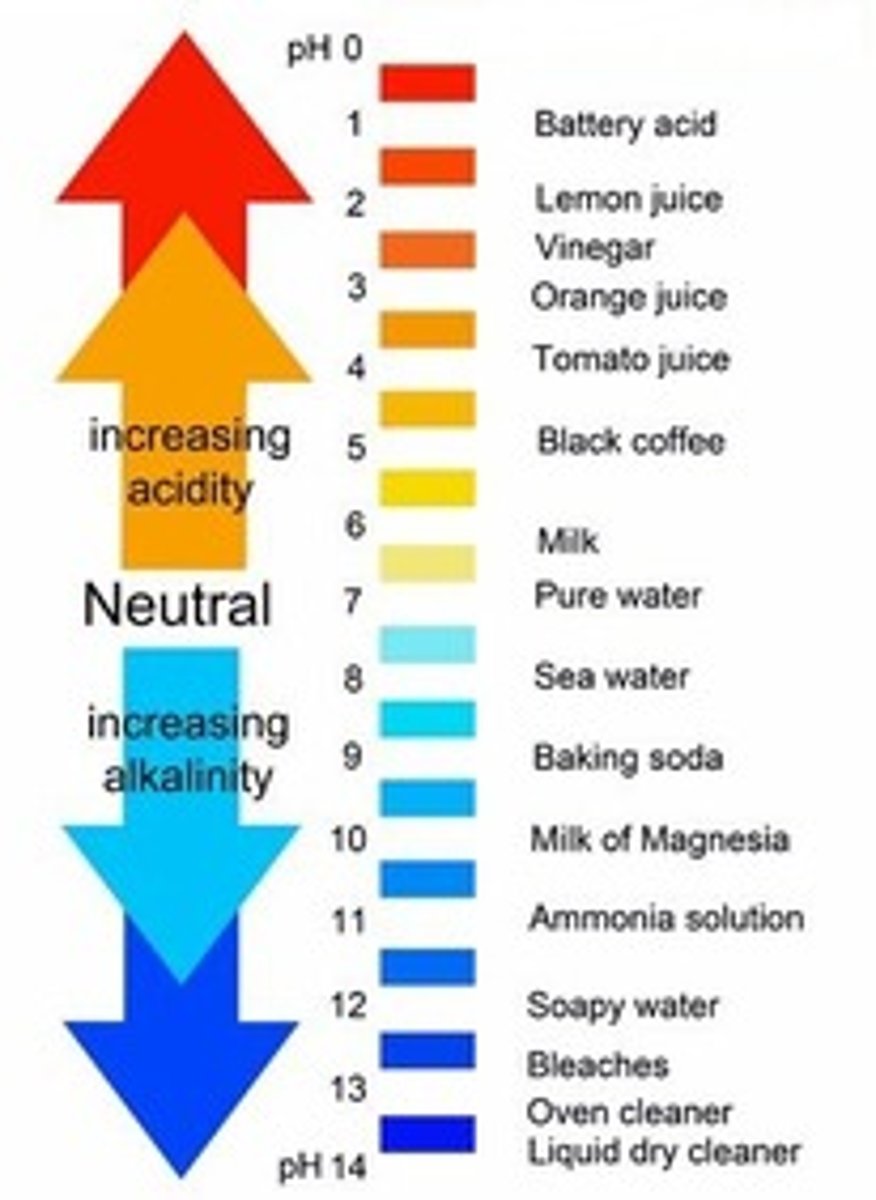
the pH of a substance
is a unitless number between 0 and 14 that tells how acidic or basic a substance is
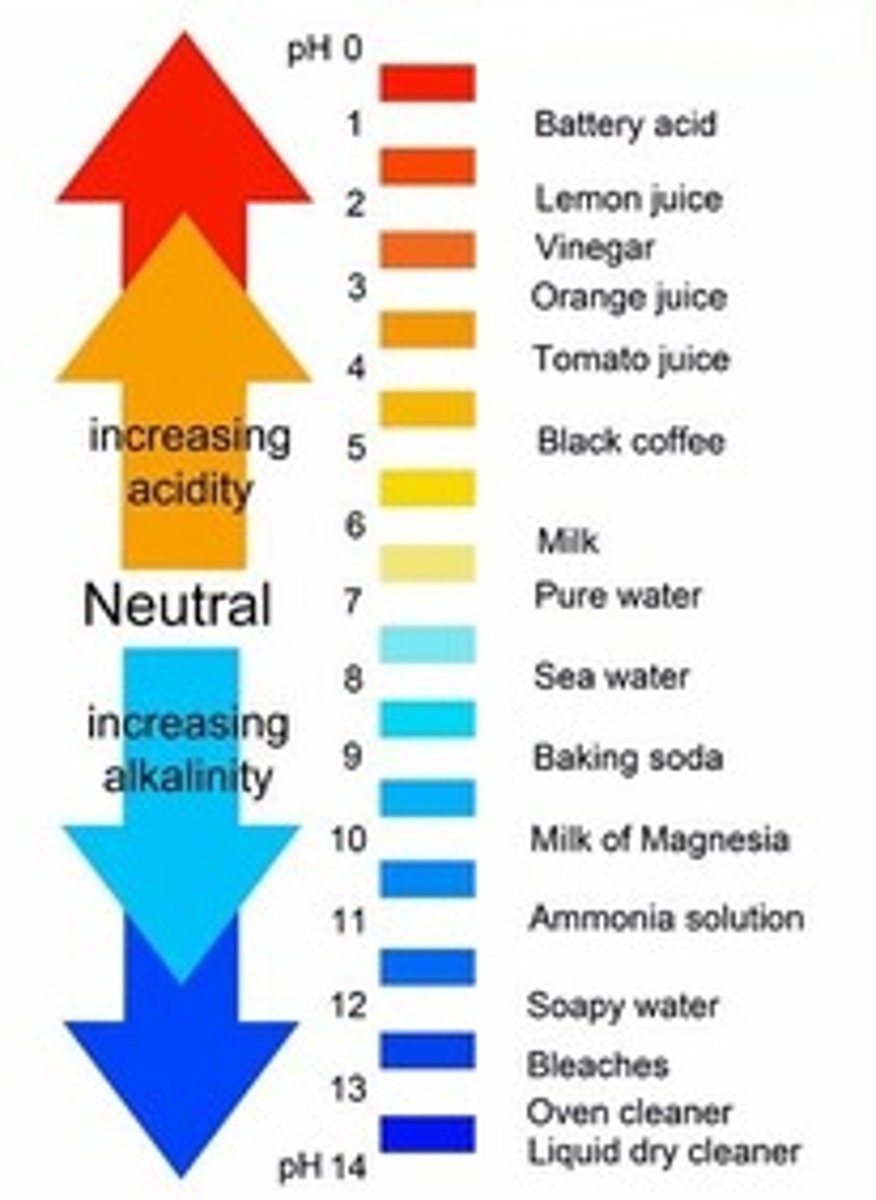
pH value is determined by
the abundance of hydrogen ions that are produced in a solution of the substance
A an acid with a pH of 1 has _____ times as many hydrogen ions as an acid with a pH of 2
10
Buffers
weak acids and bases in solution that respond to added acid or base to maintain an almost constant pH
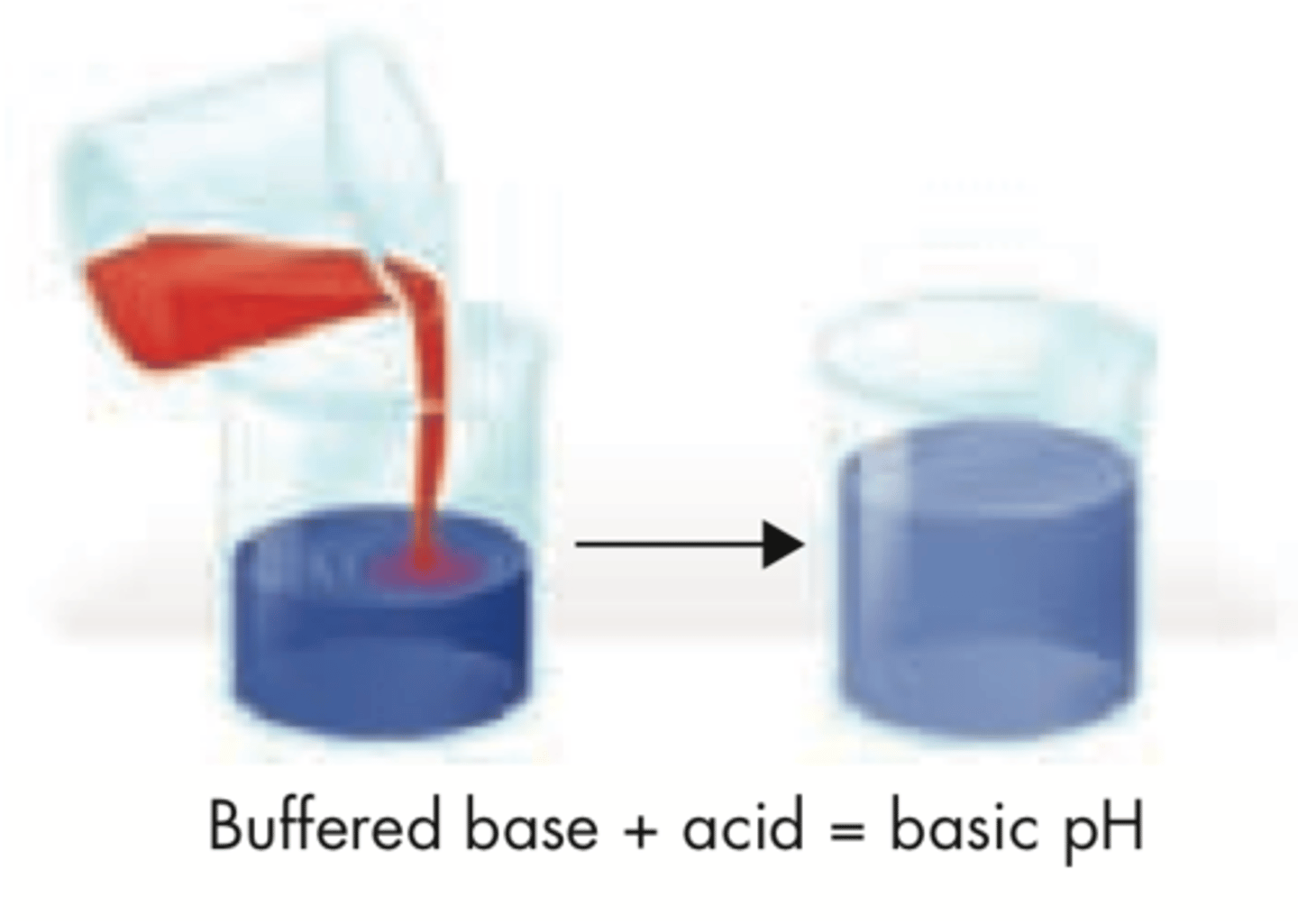
Example of a buffered system
our blood
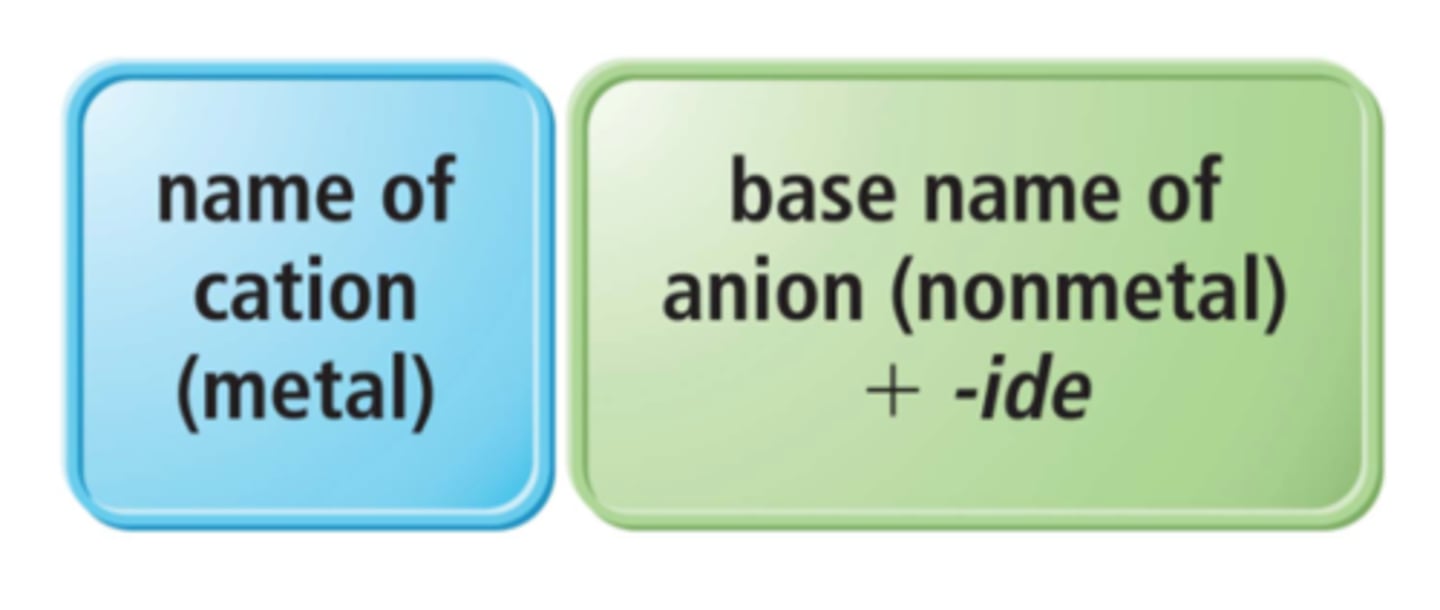
salts
electrically neutral, ionic compounds formed by a combination of cations and anions

binary salts
metal(cation) + nonmetal(anion)
Magnesium Sulfate
Epsom salt
Ammonium Dichromate
used this alt in a process called gum bichromate photography
calcium carbonate
the main component of pearls and seashells

calcium carbonate in the form of limestone
stone structures and to make concrete

malachite green
a member of a large group of salts called triarylmethane dyes
malachite is used
to treat fungal diseases in aquarium fish
neutralization reaction
the chemical reaction of an acid with a base
neutralization
A double-replacement reaction that generally produces a salt and a water
acid + base =
salt + water
one way to treat too much stomach acid
antacid, a base compound used to relieve the symptoms of indigestion
proton pump inhibitors
transports H+ ions formed from water molecules within the cells that line the stomach into tiny ducts in the stomach wall
stomach acid can cause
a very deadly kind of cancer