R3.2.8 Electrolytic cells
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
Electrolytic cells
Used to split ionic compounds into their constituent elements
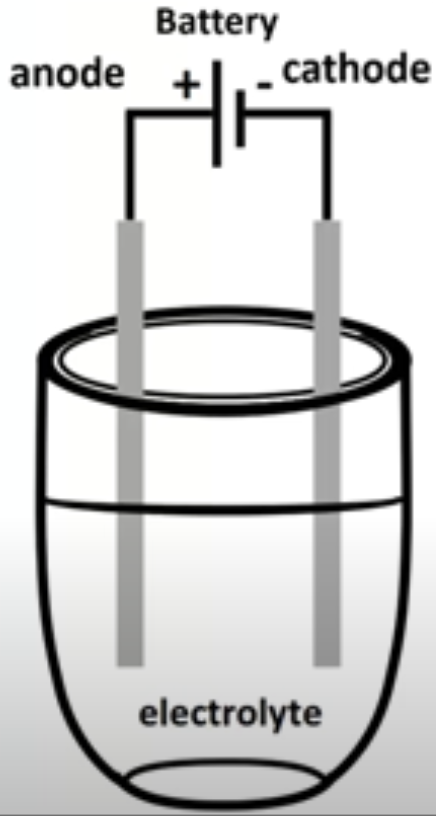
Electrolytic cell uses a single container in which an ionic compound is heated until it becomes molten
Once molten, constituent ions can move around in the electrolyte
Electric current supplied from battery, oppositely charged ions attracted to anode or cathode
Electrolysis of molten ionic compounds
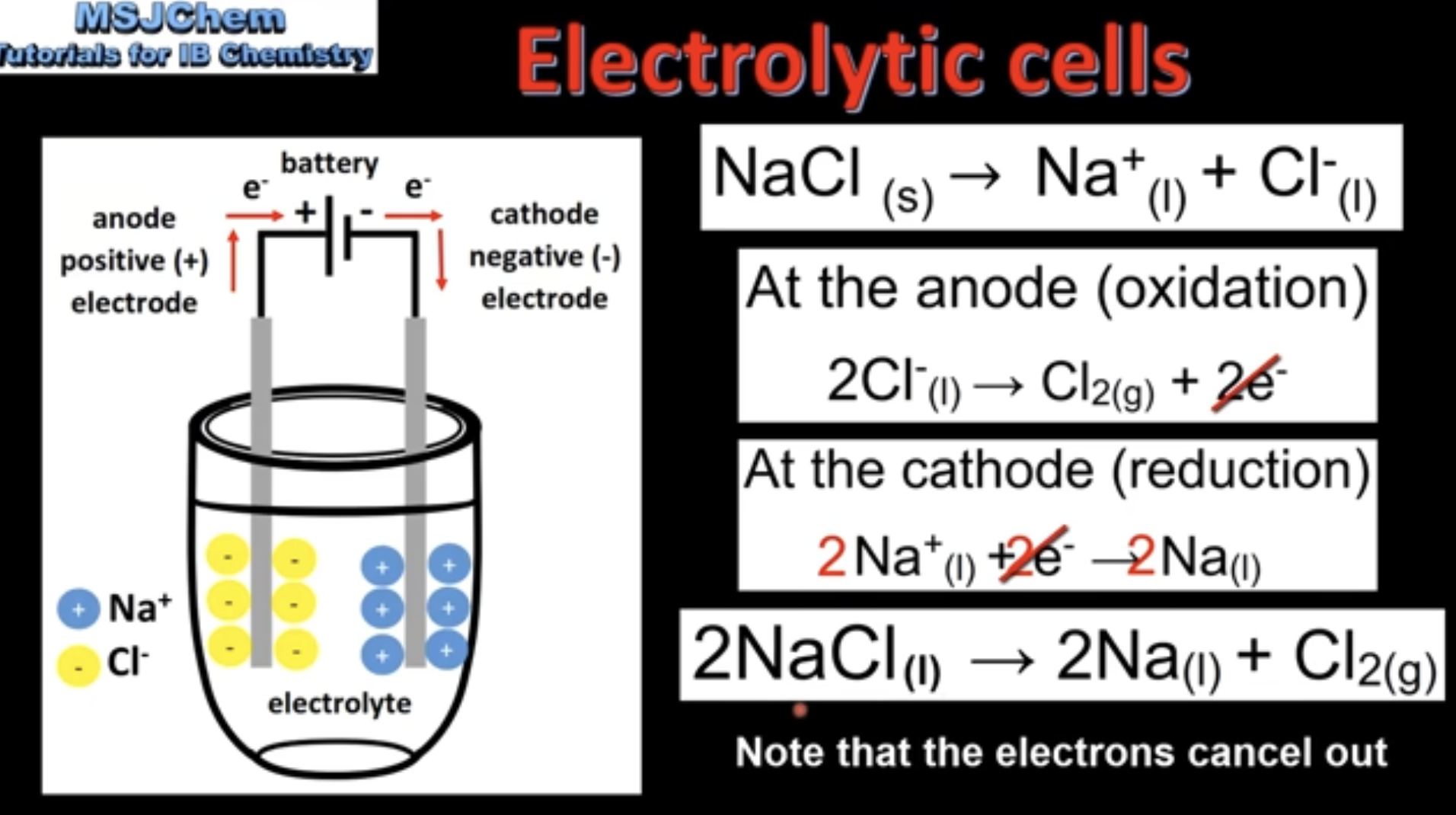
Negative ions are attracted to positive anode
Positive ions are attracted to negative cathode
At anode, negative ions are oxidized to form non-metal gas
At cathode, positive ions are reduced to form liquid metal
What must be the same between half-equations?
Number of electrons
How is an electric current conducted through an electrolytic cell?
Flow of electrons in the wires (from anode to cathode due to oxidation)
Movement of ions in electrolyte (negative ions move to cathode, positive to anode)