3.1A The Periodic Table
1/9
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
The periodic table consists of…
horizontal periods and vertical groups
Groups are labelled from…
1-18
The period number corresponds to the…
principal quantum number of the outermost electron sub levels
What is the principal quantum number?
the coeffienct before the orbital (e.g. 3s)
The people table is also divided into four blocks
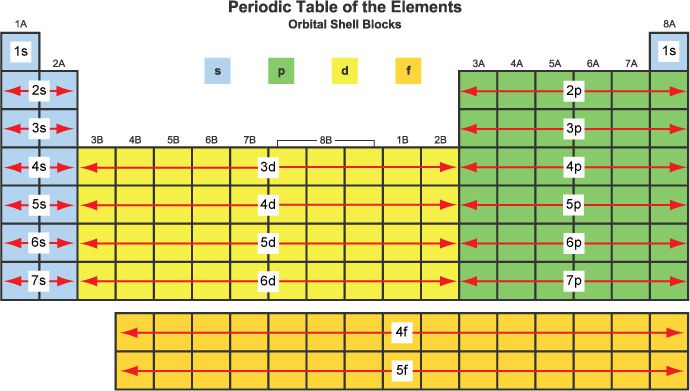
Where are non-metals found?
the upper right of the p-block
What are Halogens and where are they found?
reactive group of non-metals found in group 17
What are Noble Gases and where are they found?
very unreactive group of non-metals found in group 18
Where are metallic elements found? Tell me about their two components.
left side of p-block, the d-block, the f-block, and s-block (excluding H and He)
Alkali metals: reactive metals found in group 1
Lanthanoids and Actinides are the first and second row of the f-block
Metalloids have the physical properties and qappearance of metals (mostly), but behave in a way that is similar to non-metals chemically.
e.g. Boron, SIlicon, Germanium, Arsenic, Antimony, Tellurium, Polonium
They are found on the staircase between metals and non-metals