5: magnetic resonance
1/104
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
105 Terms
describe types of atomic/molecular interaction at different areas of EM spectrum
radio = NMR
microwave = EPR
microwave = molecular rotation
IR = molecular vibration
UV/Vis = valence e- excitation
x-rays = core e- excitation
the type of EM radiation is dependent on the size of the energy gap of transition being probed
describe NMR vs EPR
NMR = spectroscopy of nuclear spin states in a magnetic field
EPR = spectroscopy of electron spin states in a magnetic field
describe magnetic vs electric dipoles
electric dipoles = arise from two equal and opposite electric charges separated by a small distance
magnetic dipole = arise from spinning charged particle generating an magnetic field
describe NMR/EPR vs other types of spectroscopy
other = electric dipole moments interacting with electric component of EM radiation
NMR/EPR = magnetic dipole moment (electron/nucleus) interacting with magnetic component of EM radiation
describe classical angular momentum
an object rotating around a point has angular momentum
= vector = magnitude and direction
two types
spin AM = mass spinning on axis
orbital AM = mass orbiting a central point
describe the application of the rigid rotor model
to model angular momentum in rotating diatomic molecule
describe the difference in angular momentum between molecules and elementary particles
molecules = acquire rotational angular momentum (by collisions)
elementary particles = intrinsic angular momentum (spin)
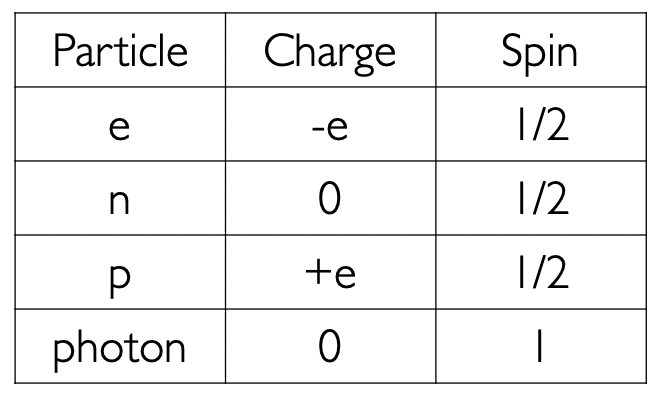
what are charge and spin for the elementary particles
describe how to differentiate vectors and quantum numbers
vectors = bold
quantum numbers = italics
describe nuclear spin
nuclear spin = intrinsic angular momentum = I
since it is angular momentum:
= vector = magnitude and direction
what equation defines the magnitude of I? (given)
I = spin quantum number
hence, the magnitude of spin is constant for a given nuclei
only direction can be changed
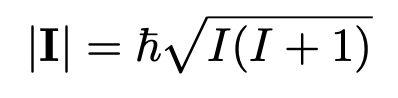
describe spin quantum number
unique to a given nuclei
= determined primarily by number of protons/neutrons
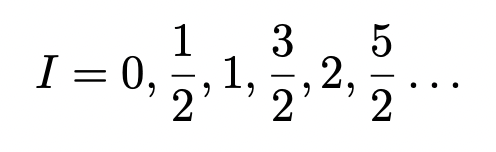
what nuclei are NMR active?
I > 0
= odd mass number
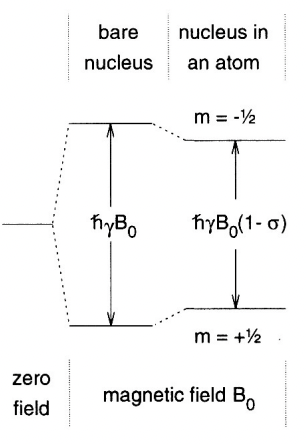
describe Zeeman splitting
a nuclei with spin I is (2I + 1) degenerate due to m(I)
application of magnetic field breaks the degeneracy
gives rise to ‘Zeeman eigenstates’
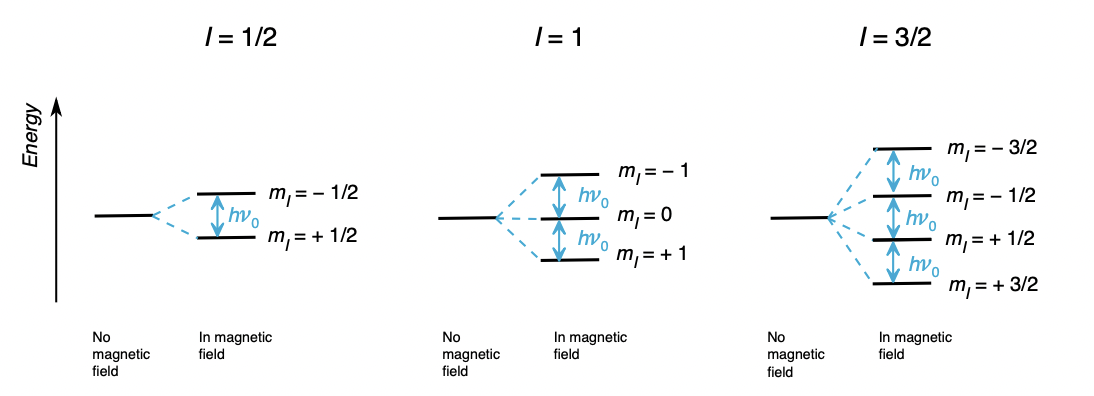
describe m(I)
m(I) = nuclear azimuthal/magnetic quantum number = describes orientation of nuclear spin (vector) in a magnetic field
= -I, -I+1, …, +I
what equation defines the energy of the Zeeman eigenstates?
m(I) = controls number of Zeeman eigenstates
γ = gyromagnetic ratio = unique for a nucleus = controls extent of Zeeman splitting (size of E gap between eigenstates)
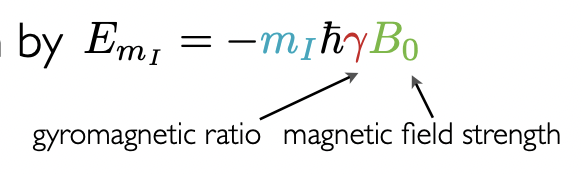
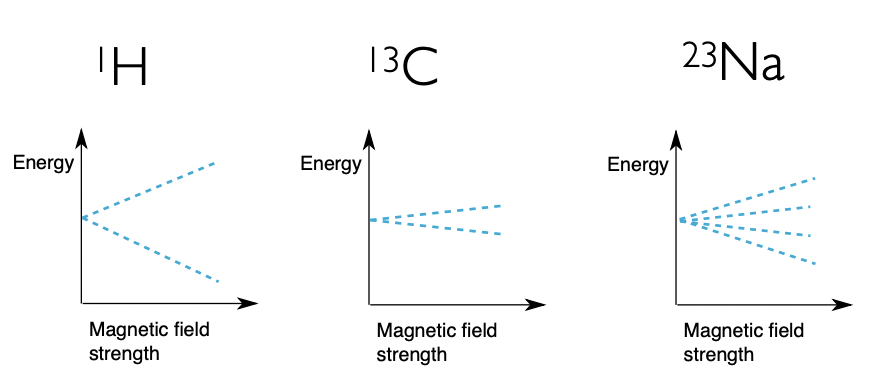
describe what varies the Zeeman eigenstates here
(1)H and (13)C:
= same I = same m(I) = both split to 2 sublevels
= different γ = different energy gaps
(13)C and (23)Na:
= similar γ = similar energy gaps
= different I = different m(I) = split into different no. of sublevels
what are the selection rules for a nuclei’s NMR activity?
I > 0 (odd mass number)
Δm(I) = ± 1
describe the energy difference between allowed Zeeman eigenstates
(using m(I) = ½ as basis)

describe the Larmor frequency (conversion to v not given!!)
the frequency of the allowed transition between Zeeman eigenstates for a given nuclei
= frequency of transition between different orientations of nuclear spin (vector) in a magnetic field
(= frequency at which nuclear spins precess in a magnetic field)
= constant for a given nucleus
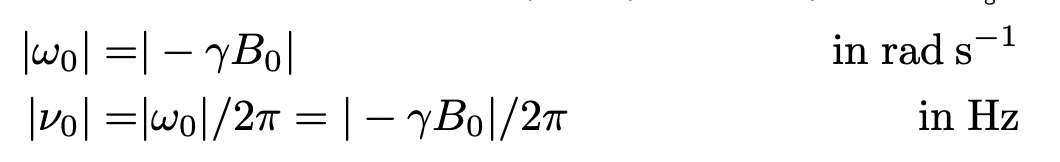
what units are B(0) in?
tesla (T)
what does the value of the Larmor frequency of many common nuclei tell us?
= fall in radio part of EM spectrum
= radio used in NMR/EPR
what is unit M (mega)?
10^6
describe the relationship between spin and magnetism
= spinning charge (internal angular momentum), I, gives magnetic moment
= magnetic moment, μ, of a nucleus is proportional to spin angular momentum, I
gyromagnetic ratio = constant of proportionality
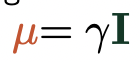
describe the possible values of gyromagnetic ratio
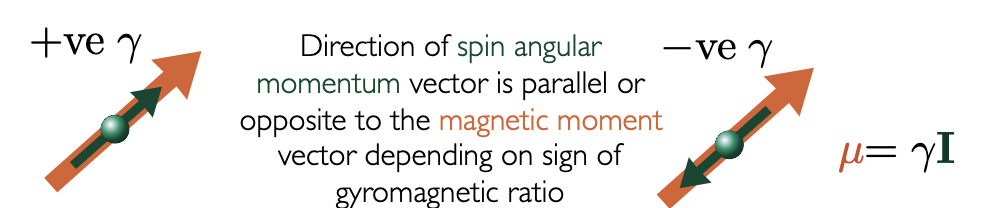
μ points in same direction as I = +ve γ
μ points in opposite direction of I = -ve γ
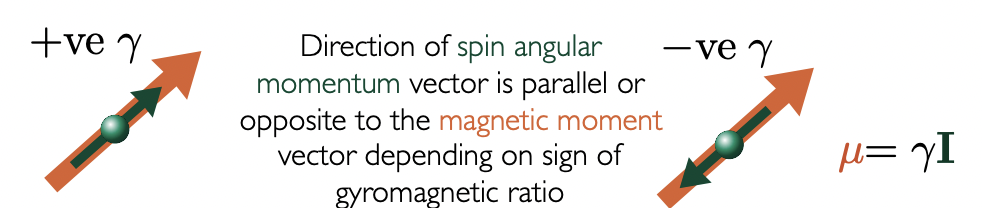
describe a and B Zeeman eigenstates
alpha (a) = ‘up’ = +ve m(I)
beta (B) = ‘down’ = -ve m(I)
describe the configuration of Zeeman eigenstate depending on values of gyromagnetic ratio
+ve γ = μ points in same direction as I
-ve m(I) (B) higher in energy (harder to flip)
-ve γ = μ points in opposite direction of I
+ve m(I) (a) higher in energy (harder to flip)
describe the problem with the energy level approach
nuclei do not have to be in just one of these two states (up or down)
= mixture of wavefunctions associated with each energy level
i.e. coherent superposition/mixed state
what is spin polarisation
alignment of nuclear spins in response to application of a magnetic field
describe the direction of spin angular momentum vector, I, with magnetic moment vector, μ
+ve γ = μ parallel to I
-ve γ = μ anti-parallel to I
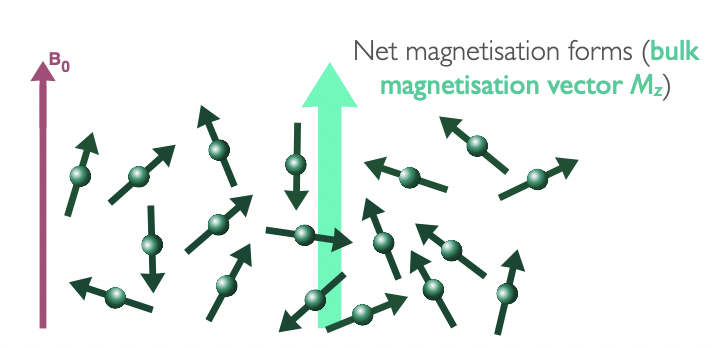
describe nuclei spins in absence of magnetic field
spin polarisation axes (spins, I) pointing in all possible direction
= no net magnetisation
what happens when a magnetic field is appled?
the spin polarisation axes (spins, I) precess around the axis of magnetic field B(0)
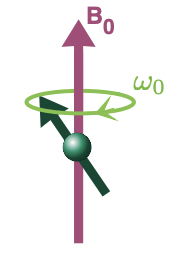
describe the frequency and angle of precession
frequency of precession = Larmor frequency
angle of precession = dependent on initial spin direction
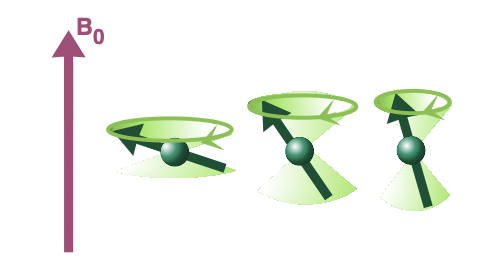
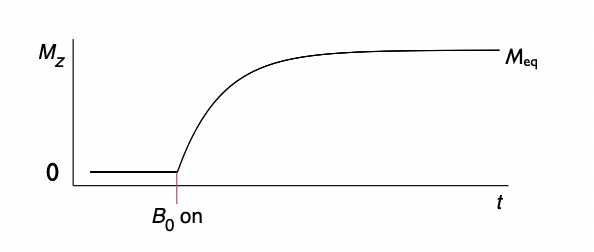
describe longitudinal relaxation
precession around axis of magnetic field = minimises energy of interaction between the spin magnetic moment and magnetic field = lower energy orientation
net magnetisation grows along z axis as spins come to equilibrium with magnetic field B(0)
= contributes to bulk magnetisation vector, M, which precesses around axis of magnetic field
draw a graph representing longitudinal relaxation
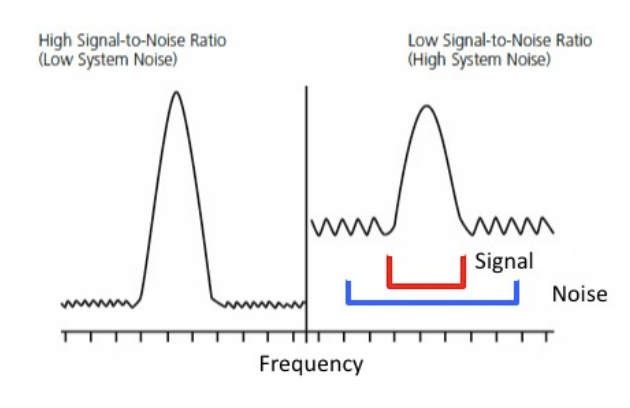
describe signal:noise ratio
high signal:noise ratio desired
describe population distributions between Zeeman eigenstates
remember ΔE = h|v(0)|
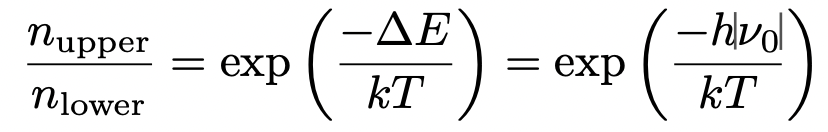
describe how population differences lead to bulk magnetisation vector
population differences between different Zeeman eigenstates = different m(I) values = different alignment to external magnetic field
n(upper) = excited state = anti aligned with B(0)
n(lower) = ground state = aligned with B(0)
the population difference is the difference in alignment to external magnetic field = bulk magnetisation vector, M
always more aligned (n(lower)) since lower energy configuration
describe the size of population differences in NMR
very small = inherently insensitive
= small bulk magnetisation vector
what increasing the sensitivity of NMR for certain nuclei?
higher gyromagnetic ratios, γ
describe detection in NMR
external magnetic field (B(0)) is applied
= creates bulk magnetisation vector = precess at Larmor frequency due to small population difference
= aligns nuclear spins at equilibrium
RF (radiofrequency) pulse at Larmor frequency tips magnetisation away from B(0) axis, causing bulk magnetisation vector M to precess in the transverse plane (xy) at Larmor frequency
precessing magnetisation creates an oscillating magnetic field that induces current in receiver coil = free induction signal (FID)
what expression describes the lifetime of the transverse component of bulk magnetisation (NOT GIVEN)
M(t) = bulk magnetisation at time t
M(0) = bulk magnetisation at time 0 (after RF pulse)
w = Larmor frequency
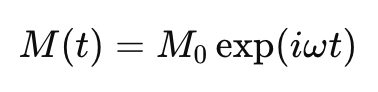
how can exp(iwt) be expressed alternatively?
using Euler’s formula
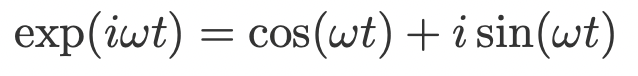
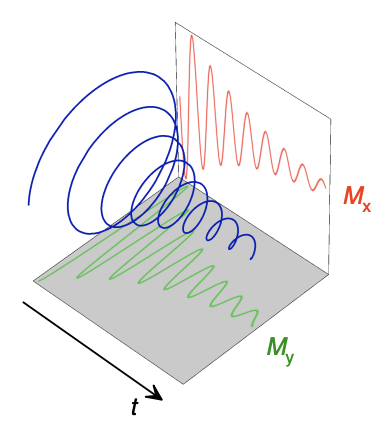
describe free induction decay (FID)
= complex and time-dependent (since transverse component of bulk magnetisation decays over time)
M(y) = real component
M(x) = imaginary component
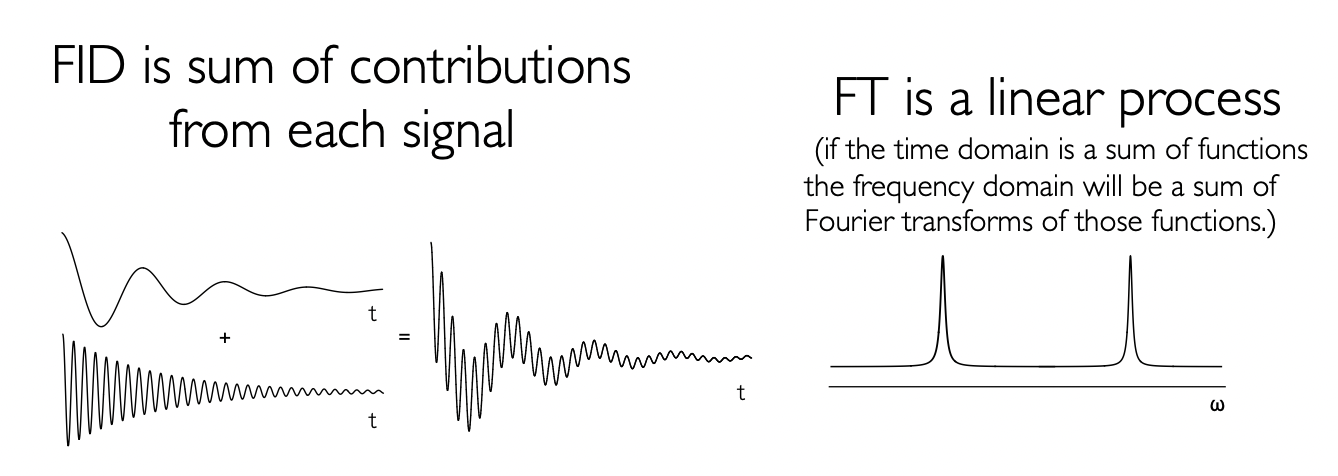
explain the application of the Fourier transform on free induction decay
to convert free induction decay (FID):
time domain signal → frequency domain spectrum
describe pulse acquisition diagrams
visualise sequence of events during NMR experiment
what process can improve SNR (signal:noise ratio)
signal averaging
describe signal averaging
experiment repeated multiple times = scans/transients
transients added up
SNR ∝ √number of transients
what is required of a magnetic to be a magnetic field in an NMR experiment?
strength
homogeneity
stability
what types of magnets can be used in NMR?
permanent (<1.5 T)
electromagnetic (<2.5 T)
superconducting (<23.5T) best on all criteria
describe NMR probes
contain rf coils (applied rf pulse AND receives signal)
houses the sample
can spin and vary temp
describe continuous wave (CW) and Fourier transform (FT) NMR
CW NMR: either
keep B(0) constant and vary frequency of rf pulse
keep frequency of rf pulse constant and vary B(0)
FT NMR:
range of rf pulse frequencies applied at once
what are the components of the electric/magnetic field experienced by a nuclear spin?
external field
the sample itself (internal)
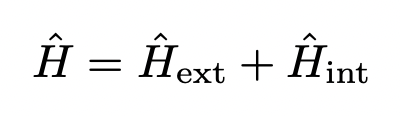
compare solution state vs solid state NMR
solution state = anisotropies are averaged
solid state = anisotropies are NOT averaged = more complex
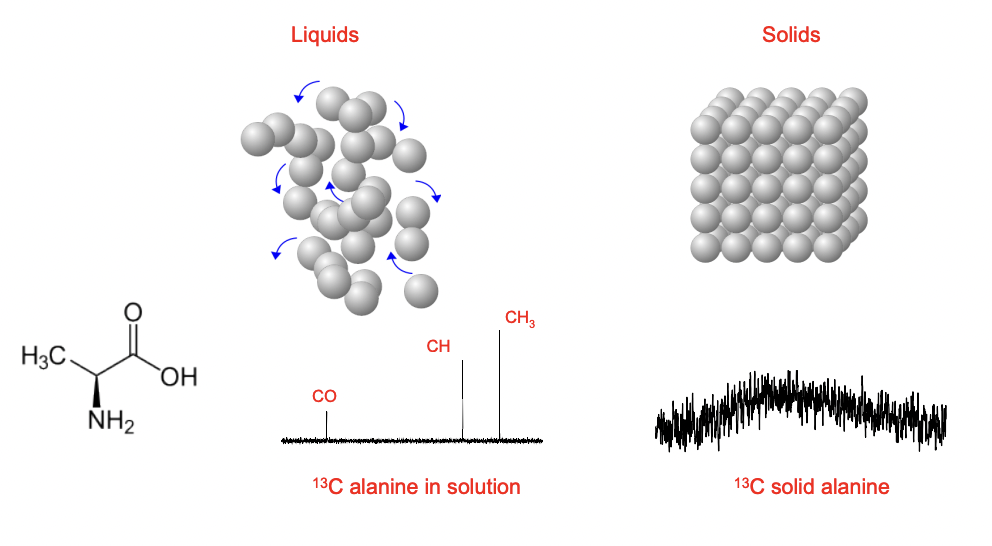
describe the effect of a nuclei’s local distribution
the local electron distribution around a nucleus effects the resonance frequency at which it processes around the external field
theory = 1H at B(0) = 9.4T should resonate at 400MHz
B(in) = induced magnetic field from external magnetic field caused by circulation of electrons in their AO/MOs
B(in) either opposes (shields = lowers B) or augments (deshields = increases B) the external field
B = effective field
define effective field at nucleus
B = effective field
B(0) = external magnetic field
B(in) = induced magnetic field
σ = shielding
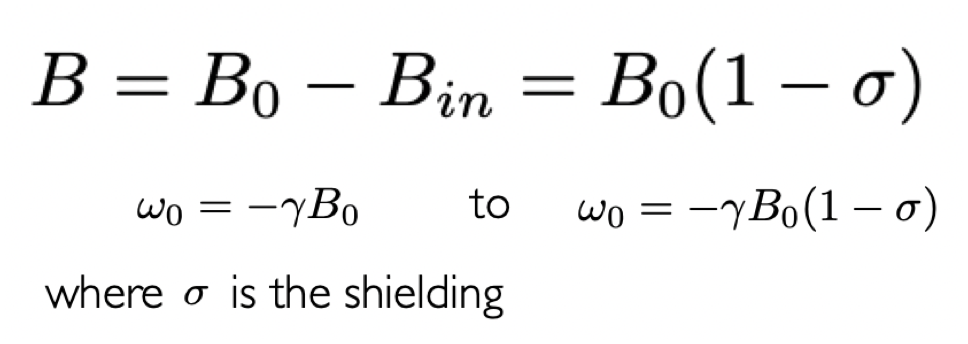
describe the effect of shielding
shielding reduces the Larmor frequency of a nucleus
= reduces the energy gap between Zeeman eigenstates
define chemical shift (given)
( / v(spectrometer) ) removes the dependence on the external field
defines the difference in Larmor frequency of a nucleus w.r.t. a reference
= define the extent of shielding

describe shielding in atoms vs molecules
atoms = simple = only diamagnetic shielding
molecules = complex (motion of electrons more complex) = diamagnetic and paramagnetic de/shielding
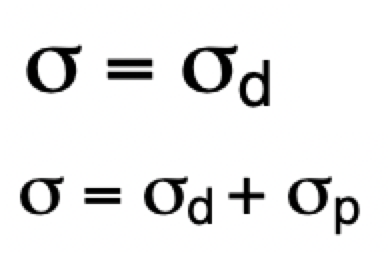
describe diamagnetic and paramagnetic de/shielding
diamagnetic shielding = reduces the strength of the magnetic field = induced magnetic field opposing B(0)
= circulates with atomic and molecular orbitals
paramagnetic deshielding = increases the strength of magnetic field = induced magnetic field in the same direction as B(0)
= circulates between occupied ground state MO and unoccupied excited state MO
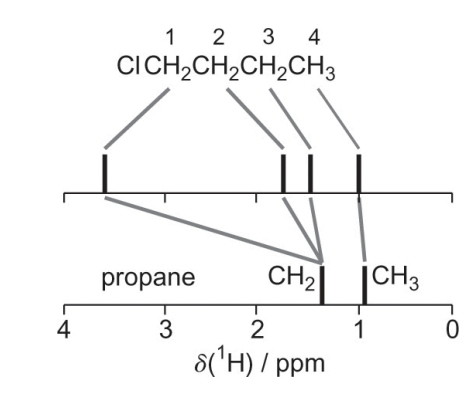
describe the chemical shifts here
diamagnetic shielding effect
aliphatic = circulating electron create B(in) = decrease B(0) = small ppm
Cl = EWG = reduces the shielding/B(in) = larger B(0) w.r.t aliphatic = large ppm
what are different “anomalies” of chemical shift?
ring current
H bonding
paramagnetic compounds
anisotropy
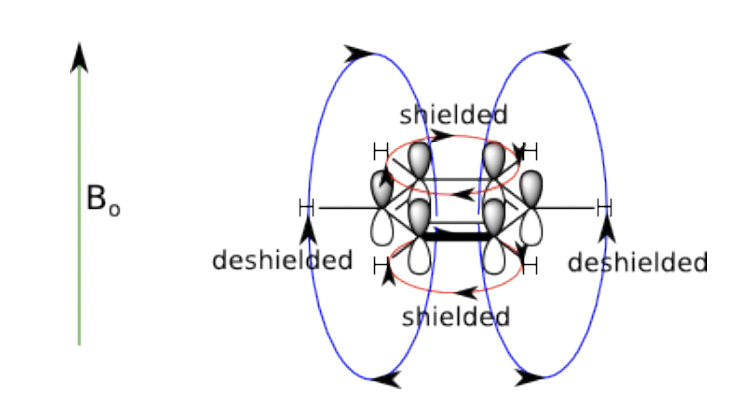
describe ring current
= aromatics
= ∏ electron clouds generate large electronic currents opposing/aligning with external field
downfield = larger chemical shift = larger B(0) than expected = smaller B(in) = ring currents align with and augment B(0)
= shielding effect directly above and below the ring
= deshielding effect in the external plane of the ring
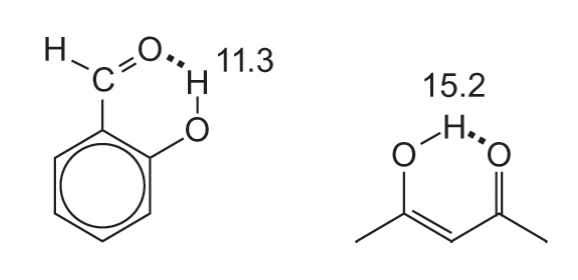
describe hydrogen bonding
= largest (1)H chemical shifts
intramolecular » intermolecular
H bond acceptor draws H away from the heteroatom it is bonded too, reducing the electron density around the H nuclei
= deshielded = smaller B(in) = larger B(0) = larger v(signal) = larger ppm
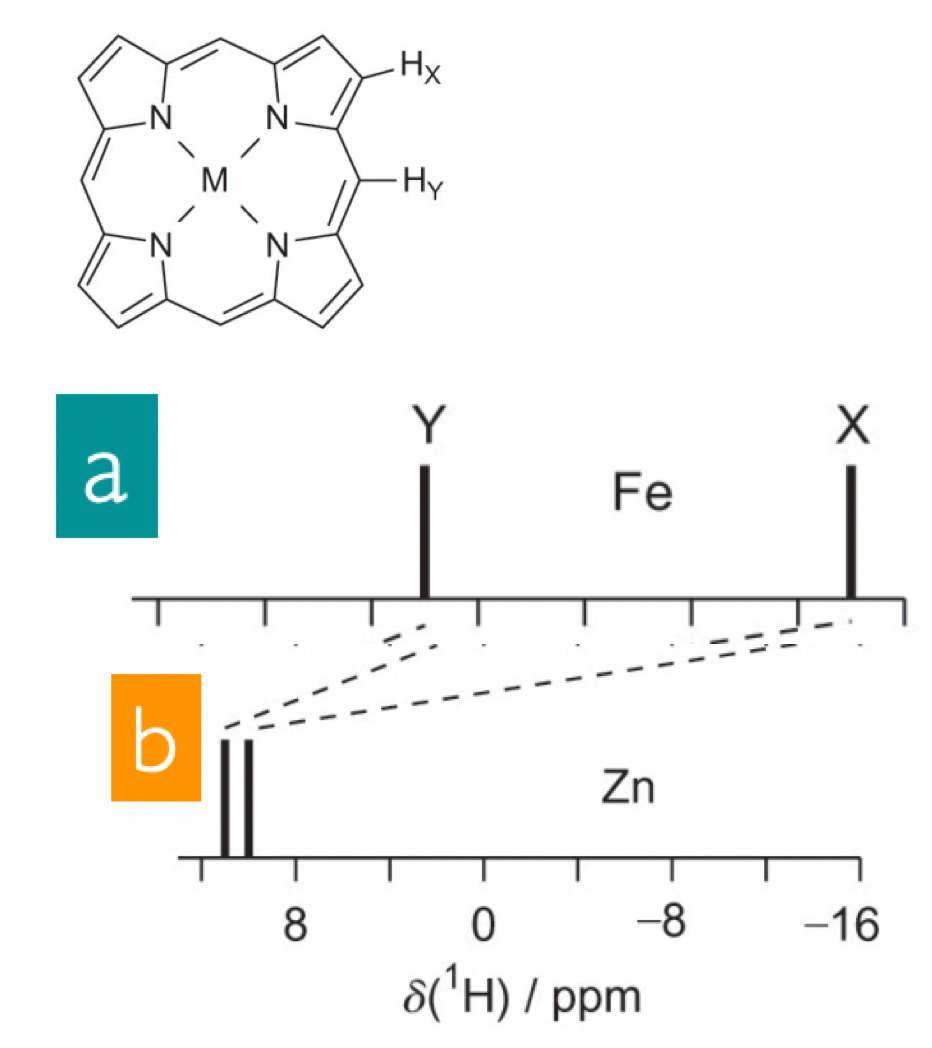
describe paramagnetic compounds
compounds with unpaired electrons generate magnetic field in the presence of external magnetic field
= increase B(in)
= smaller B(0)
= lower ppm = upfield shift
describe anisotropy (CSA = chemical shift anisotropy)
electron density is anisotropic = shielding is anisotropic = orientation dependent
liquids = rapid tumbling = anisotropic averaging
solids = different orientation of molecule = different amount of shielding
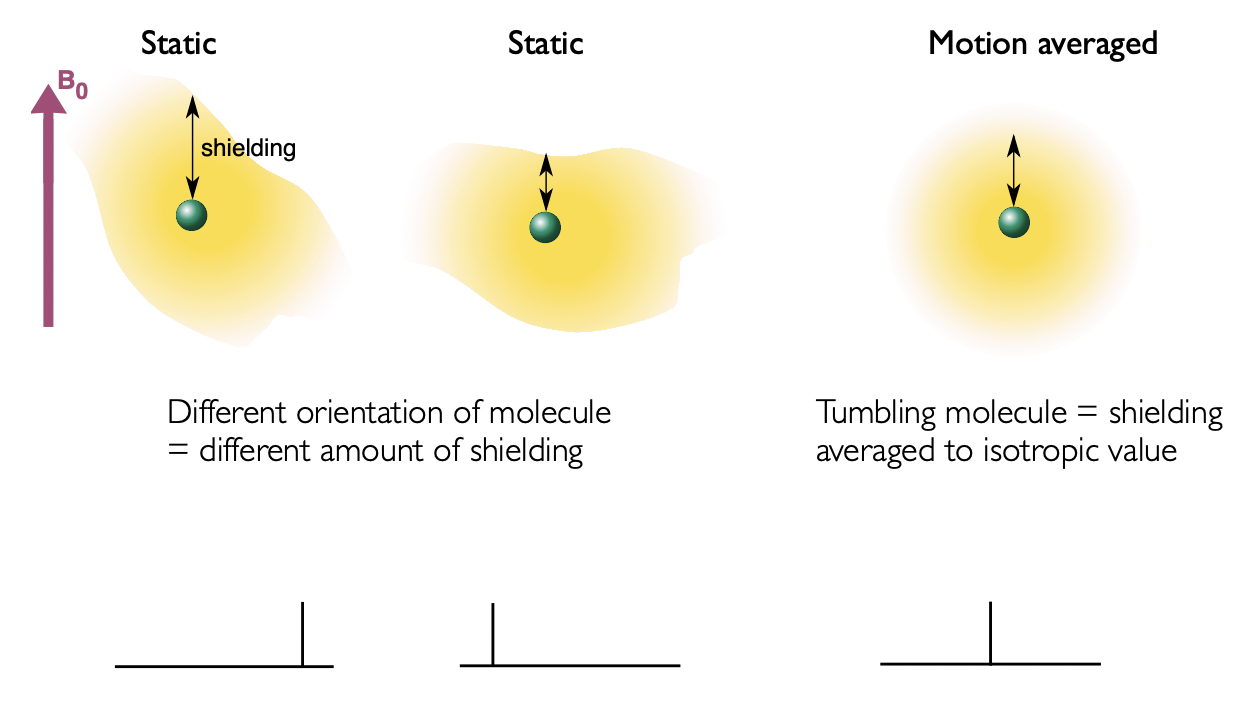
describe CSA in single crystals
single crystals:
= all molecule have the same orientation w.r.t. applied field = equivalent anisotropy = single shift
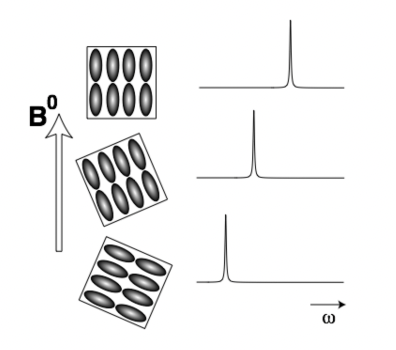
describe CSA in powders
powder:
= millions of randomly orientated single crystals w.r.t. applied field = different shifts
static powder = broad peak
isotropic (all directions) rotation = CSA averages out = single isotropic chemical shift = sharp peak
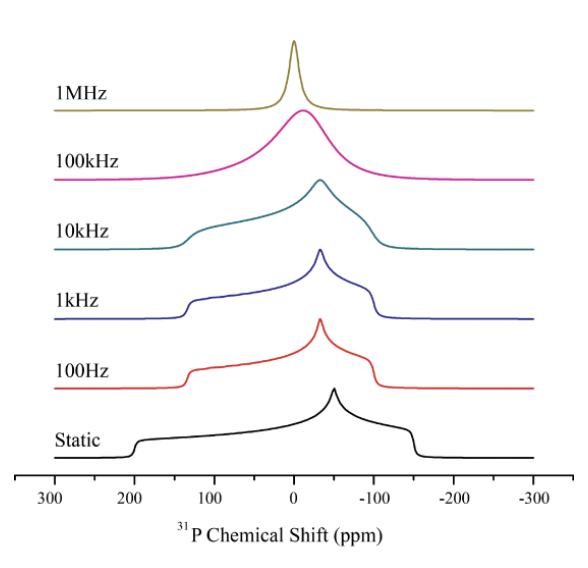
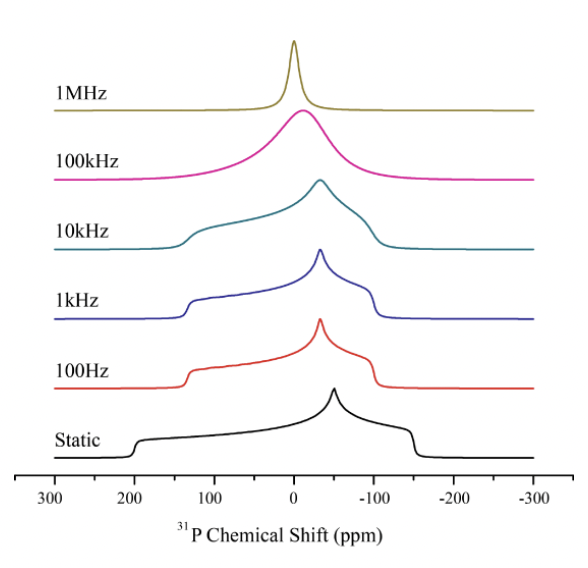
how can the CSA be described here?
Δ = 200ppm ?
δ(iso) = 0 = isotropic chemical shift
η = asymmetry of the chemical shift tensor (the variation from δ(iso)) = 0.5
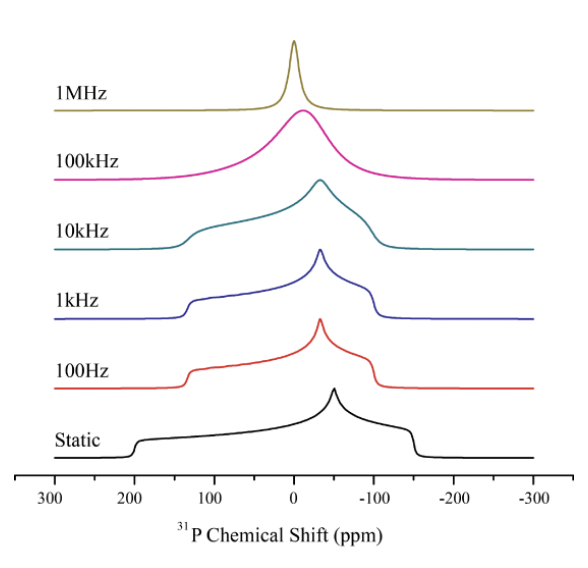
describe dipolar interactions
= through space coupling
= magnetic moment (from spin) created when nuclei/unpaired electrons are placed in magnetic field can interact
= interaction of magnetic moments leads to splitting as there are multiple different arrangement of moments with different energies
i.e. parallel = lower E; anti-parallel = higher E
dipolar interaction ∝ gyromagnetic ratio (∝ magnetic moment)
dipolar interaction ∝ 1/r (distance between nuclei)
dipolar interaction related to θ (angle between nuclei)
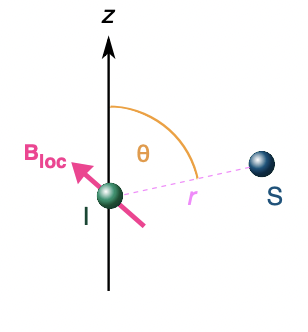
describe dipolar interactions in solution vs solids
solutions = orientations of magnetic dipoles averaged = no interaction
solids = orientations of magnetic dipoles constant = interaction
split by the dipolar coupling constant
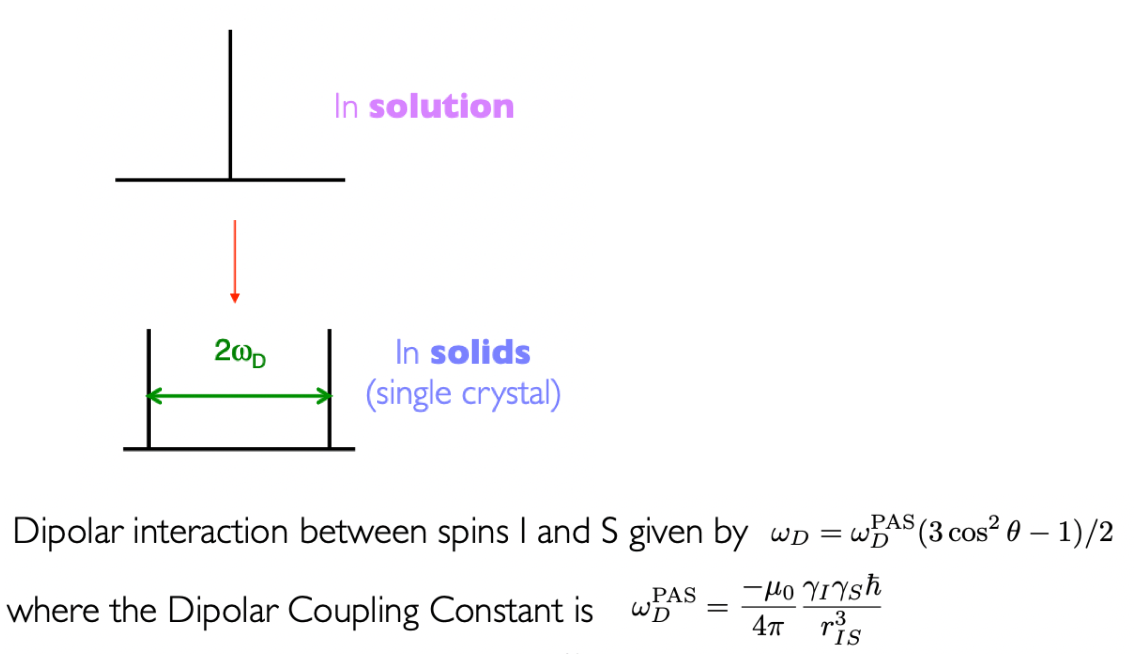
describe Pake Doublet
result of dipolar interaction in solid powder samples
powder sample = average of all possible magnetic moment orientations is observed (since magnetic moment/spin is nuclear property not structural)
= varying angles of θ = Pake doublet peak follows cosθ
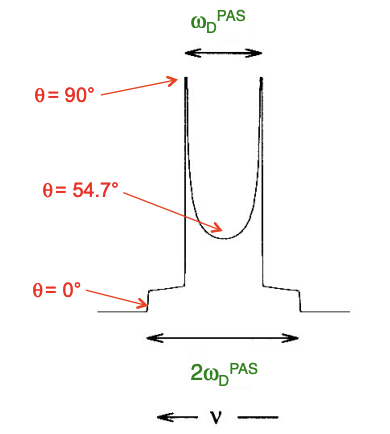
compare powder chemical shift anisotropy vs dipolar interaction anisotropy
CSA: static = broad peak
isotropic rotation = sharp single peak
dipolar interaction anisotropy: static = average = Pake doublet
describe J-coupling
= through bonds
= coupling of pairs of nuclear spins indirectly through electrons in bonding orbitals
= isotropic since through bonds
= independent of B(0) since I does not depend on B(0)
= very weak w.r.t. v(signal) ~10Hz
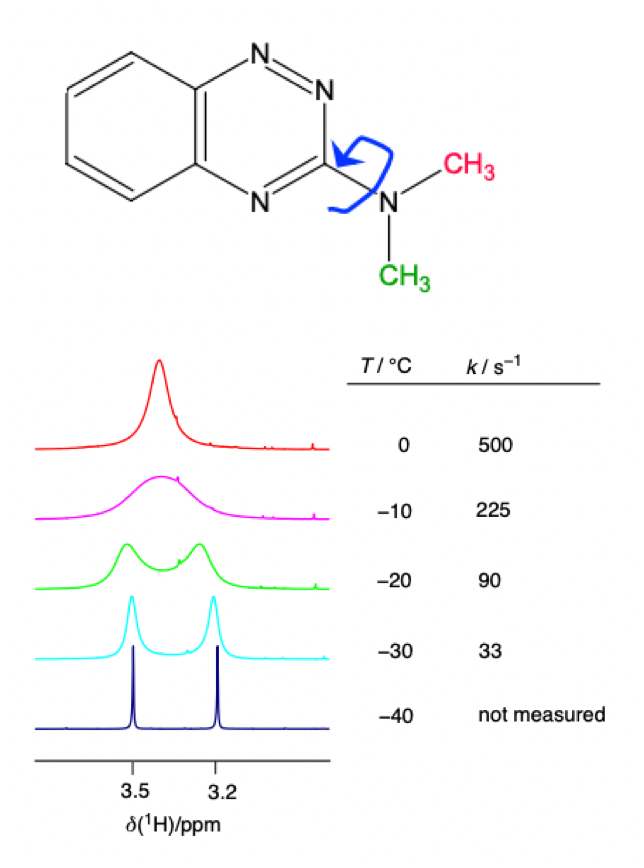
what is the condition for weakly vs strongly coupled nuclei
weakly: difference in Larmor frequencies greatly exceeds their mutual coupling
strongly: difference in Larmor frequencies similar to their mutual coupling
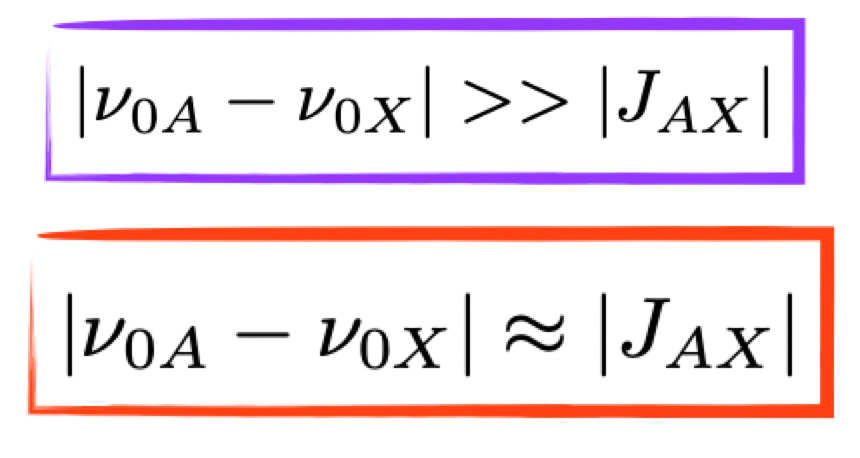
what are the options for coupling of a pair of spin = ½ nuclei?
A = weakly coupled = 2 doublets
B = strongly coupled = intermediate = ‘second-order’ appearance = roof effect
C = magnetically equivalent = 1 singlet
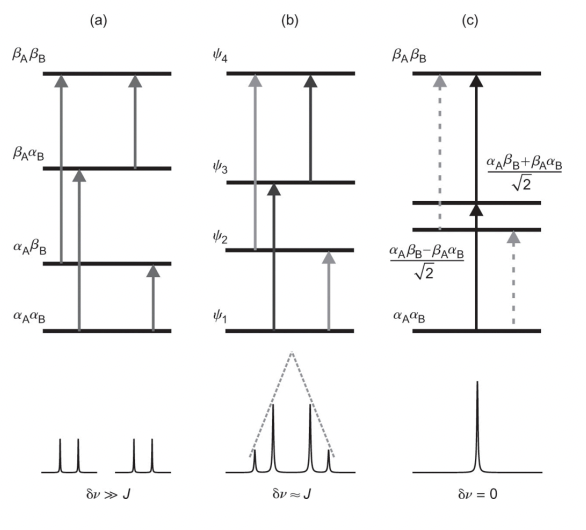
explain multiplet patterns in spin I = 1/2
the number of possible arrangement of spins depends on: I = spin
2I + 1, where I = spin
1 nuclei: (I=1/2)
2(1/2) + 1 = 2
2 coupled spins can either be parallel or anti-parallel. this gives rise to a doublet peak.
2 x 1 inequivalent nuclei: (I=1/2)
2(1/2) + 1 = 2
2(1/2) + 1 = 2
doublet of doublets
2 x equivalent nuclei: (I=1/2)
total I = 1: 2(1) + 1 = 3
triplet
coupling to 1: 2(1/2) + 1 = 2 doublet
coupled to 1: 2(1/2) + 1 = 2 doublet
~doublet of doublets with coinciding central line = triplet
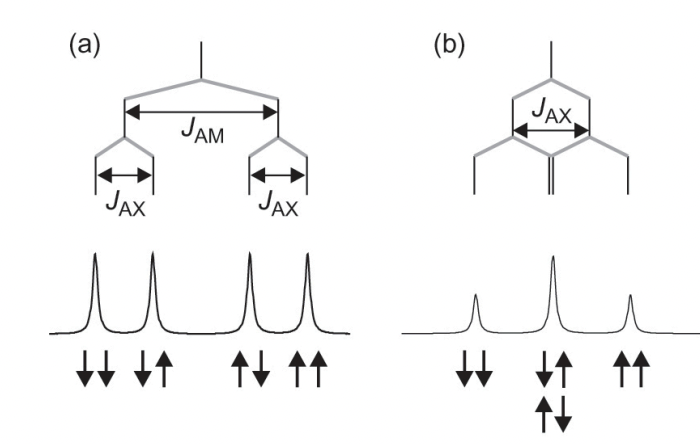
explain multiplet patterns in quadrupolar nuclei (I > 1/2)
= typically obscured by quadrupolar interaction
pattern should follow 2nI + 1
n = number of equivalent spin I nuclei 2
process:
find multiplicity by 2nI+1
find pattern to 1 by 2I+1
repeat for each equivalent nuclei

describe the two types of equivalence
chemical = same electronic environment
magnetic = same interaction with applied magnetic field
describe what happens when B(0) is very weak
Larmor frequency small = chemical shift is small = chance of strong ‘second-order’ appearance increases
describe features of the roof effect
intensities of doublet distorted
doublets no longer centres on chemical shift
describe quadrupolar interaction
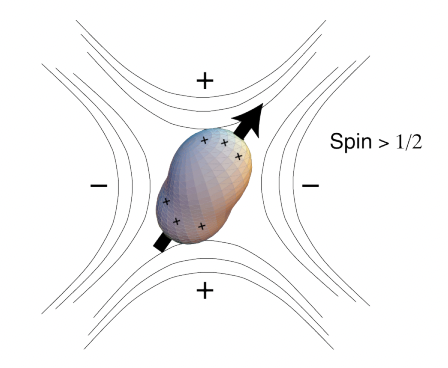
quadrupolar nuclei (I>1/2) have non-spherical distribution of charge → electrical quadrupole (eQ) moment
quadrupolar nuclei have:
magnetic moment (related to spin)
eQ moment (related to charge distribution)
quadrupolar interaction = eQ moment couples to electric field gradient (EFG) created by electron density surrounding nucleus
= very large up to 50MHz
describe quadrupolar interaction in liquids vs solids
quadrupolar interaction is anisotropic with respect to B(0) because the EFG is dependent on the direction of B(0)
liquids = rapid molecular motion = averaged = no interacton
solids = broad lines
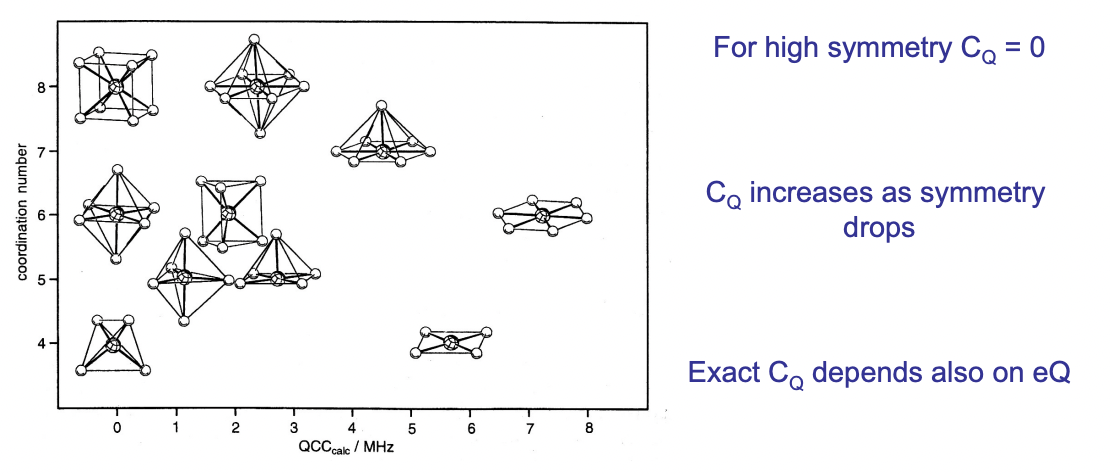
describe C(Q) and how it is approximated
C(Q) = quadrupolar coupling constant = describes extent of quadrupolar coupling
C(Q) ∝ size of EFG ∝ 1/symmetry of electron density distribution
EFG can be estimated by the symmetry of the molecule
higher symmetry = smaller EFG = lower C(Q)
lower symmetry = larger EFG = higher C(Q)
describe the appearance of a peak with quadrupolar interaction
doublet
solids = sharp peaks
liquids = averaged = Pake doublet = defined by cosθ
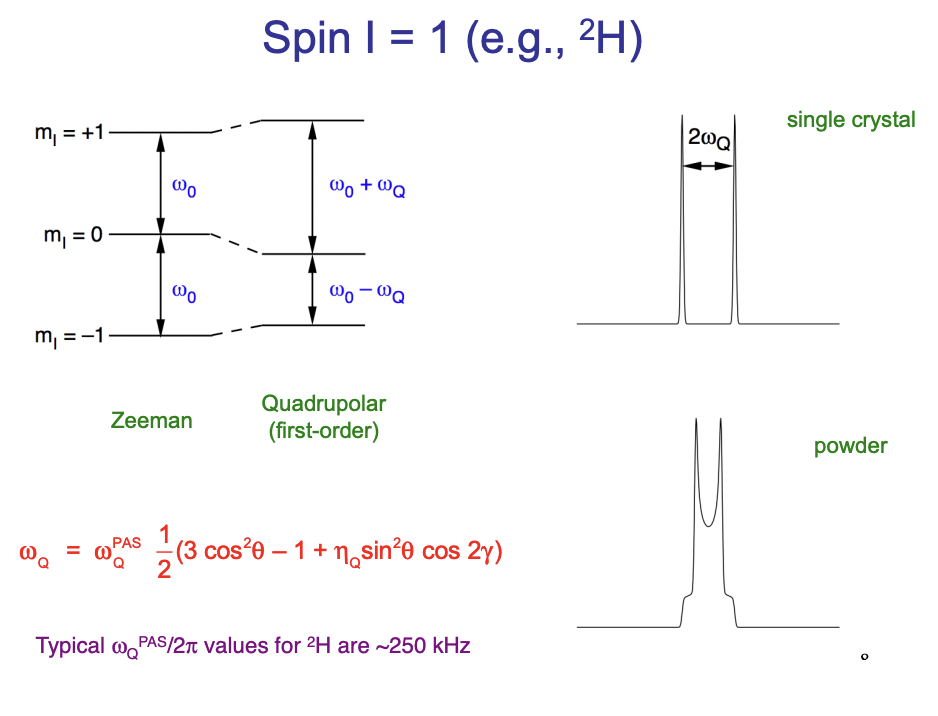
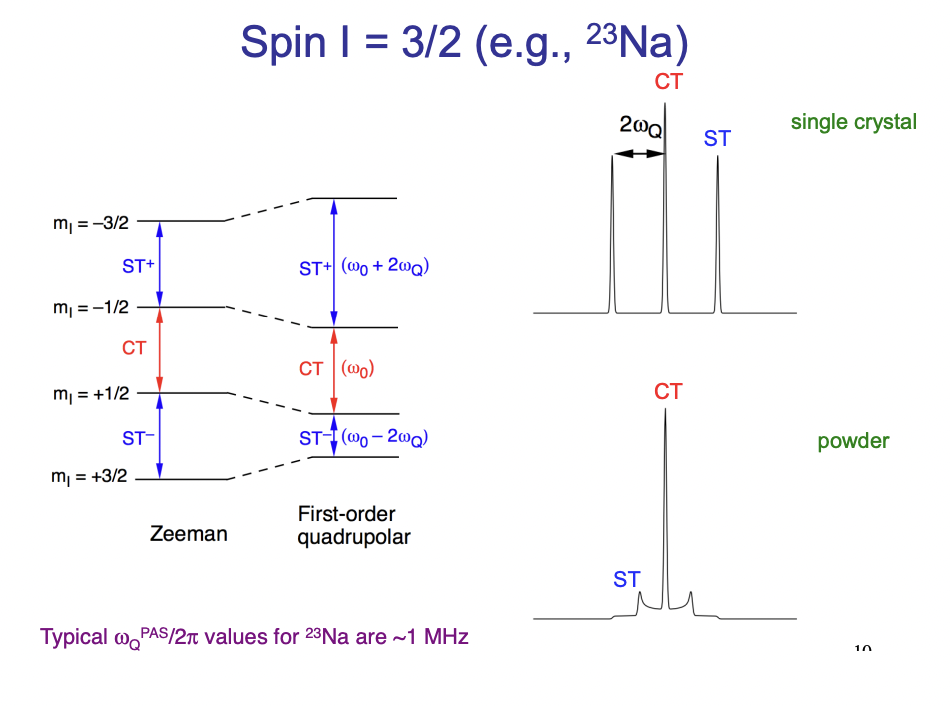
define CT and ST
CT = central transition = between 2 magnetic quantum states (+ve → -ve)
ST = satellite transition = between adjacent m(I) states (+ve → +ve etc.) = more sensitive to quadrupolar coupling
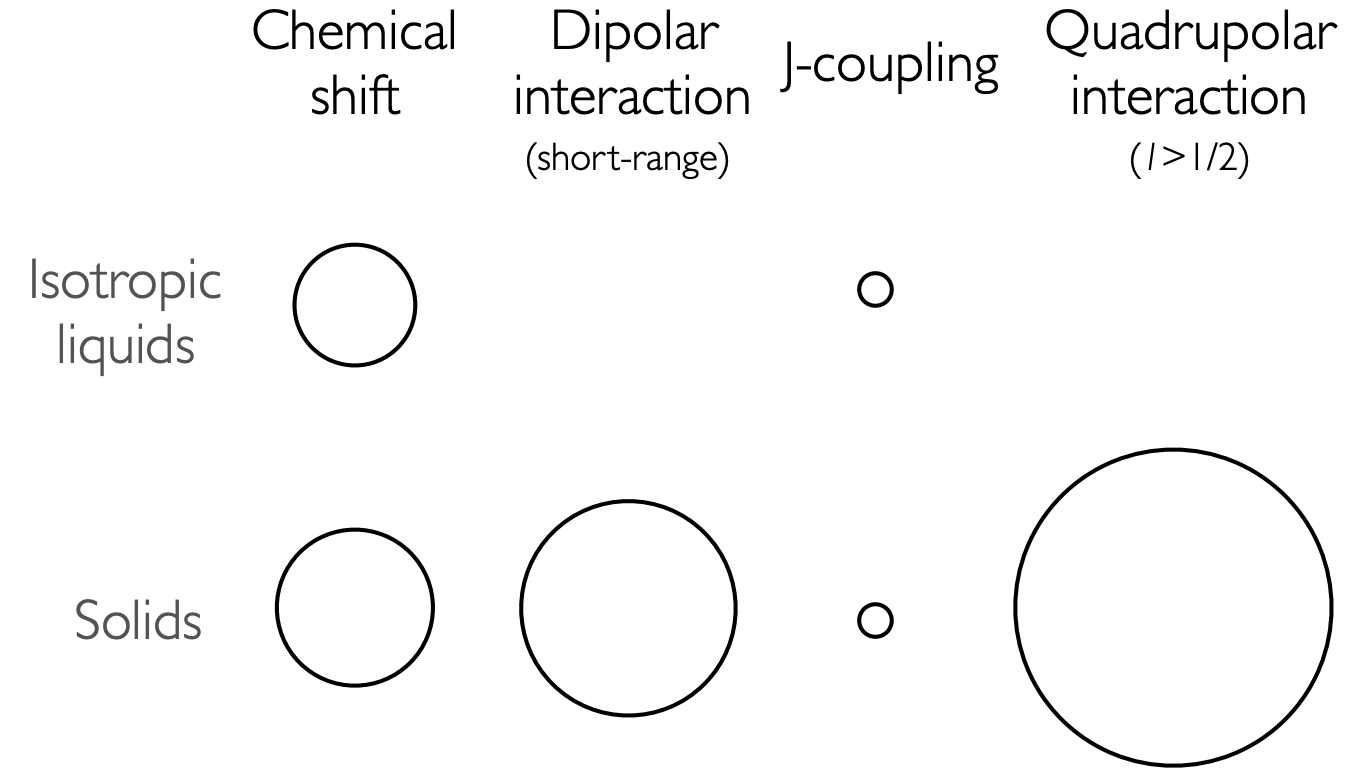
describe the relative magnitude of internal interaction in
isotropic liquids
solids
describe relaxation
spins are aligned to magnetic field B(0) along z-axis = precessing at Larmor frequency = z-magnetisation
RF pulse is applied along xy plane = x-y magnetisation
spins relax to ground state = z-magnetisation (NO xy magnetisation)
relaxation is caused by local fields which are different for each spin
what is the conditions for local field inducing relaxation
MUST be at/around the Larmor frequency
describe the two types of relaxation
spin-lattice (T1)
spin-spin (T2)
describe spin-lattice relaxation/longitudinal relaxation
z-component of magnetisation coming to equilibrium
local fields cause spin to rotate either:
TOWARDS z-axis = energy release to surroundings
AWAY from z-axis = energy investment from surroundings
equal = zero z-magnetisation
lattice is more happy to take up energy than give out energy because it is at thermal/Boltzmann equilibrium = non-zero z-magnetisation (tending towards z-axis)
M(eq) = lattice frequency
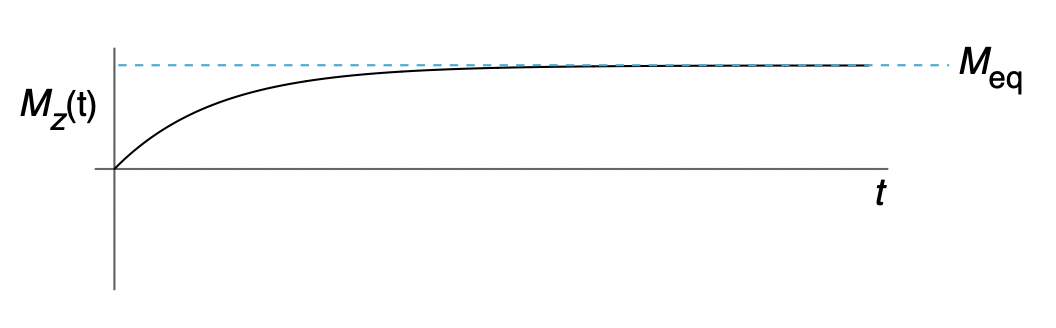
describe T1
larger T1 = longer time to reach equilibrium = slower relaxation
smaller T1 = shorter time to reach equilibrium = fast relaxation
what makes spin-lattice relaxation fast?
fast energy transfer between local fields and lattice (and vice versa)
B(loc) at the Larmor frequency from CSA/
what are origins of local fields?
anything that causes fluctuations to local magnetic field (stronger) increases relaxation = more chance of approaching Larmor frequecy
dipolar interaction = magnetic field effected to approach the Larmor frequency
∝ γ(1)γ(2) = extent of interaction
∝ 1/r3
chemical shift anisotropy (CSA) =
anisotropic nuclei experience slightly different B(0) = B(loc) approaches Larmor frequency
quadrupolar interaction
very fast relaxation is C(Q)/quadrupolar interaction is large
paramagnetic species
unpaired electrons produce large magnetic moments which can affect B(loc)
describe spin-spin relaxation/transverse relaxation
decay of xy component of magnetisation to equilibrium value of 0
spins interact with each and cause different local fields in xy plane = precess at slightly different Larmor frequencies = net xy magnetisation decreases over time
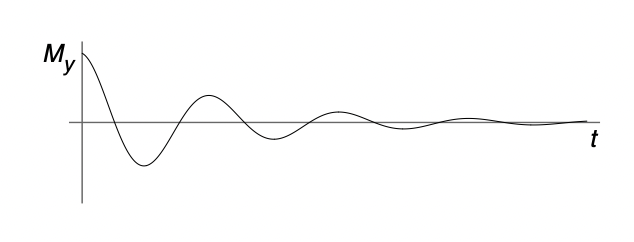
describe T2
larger T2 = longer time to reach equilibrium = slower relaxation
smaller T2 = shorter time to reach equilibrium = fast relaxation
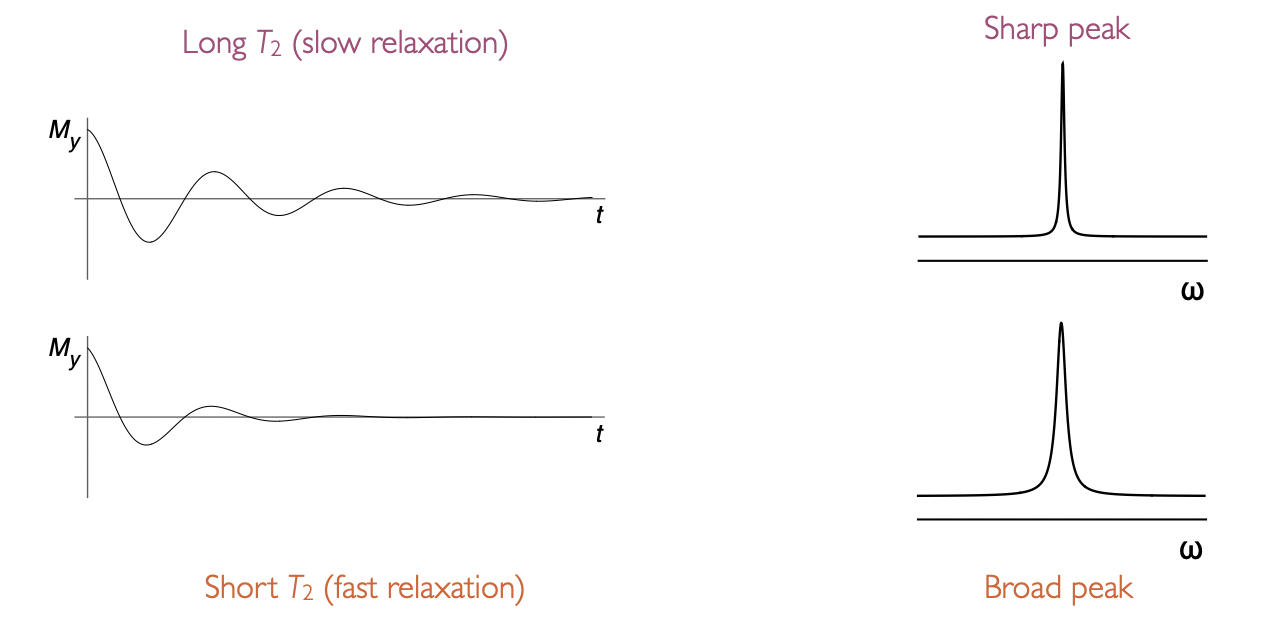
describe the appearance of peak with T2
gives linewidth
long T2 = slow relaxation = sharp peak
short T2 = fast relaxation = broad peak
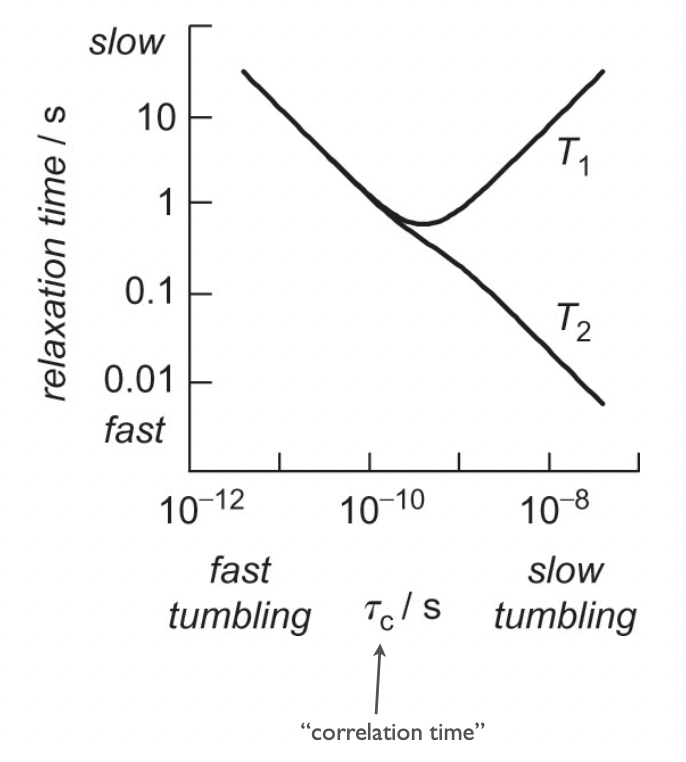
describe this graph
low τ(c) = fast molecular vibration = liquid
high τ(c) = slow molecular vibration = solid
liquid = equal contribution of longitudinal/transverse relaxation
solid = transverse relaxation > longitudinal relaxation
T₂ relaxation in solids is much faster than T₁ because the local field fluctuations due to dipolar interactions cause rapid dephasing, but the lack of molecular motion limits the efficiency of energy transfer to the lattice (T₁).
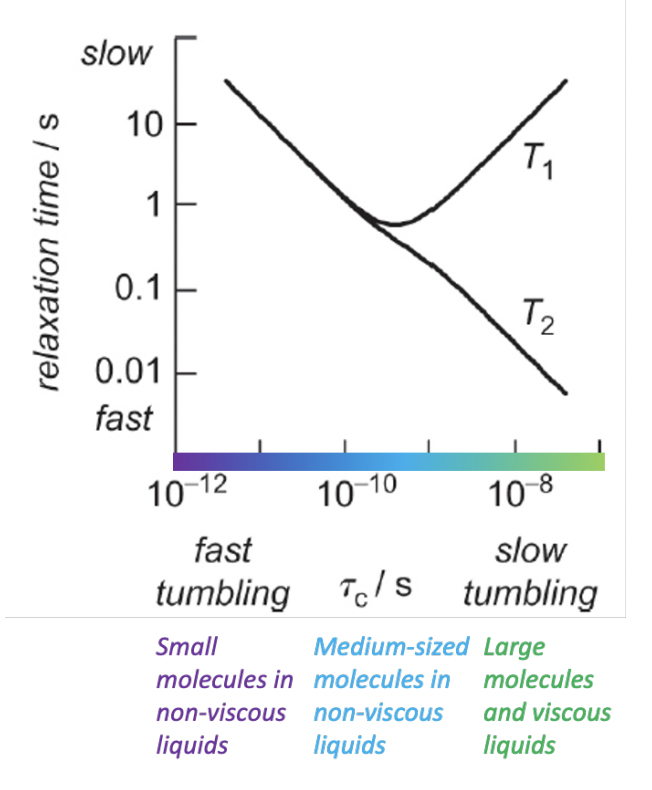
describe an application of NMR
= molecular dynamics
= can study inequivalent signals tending to equivalence due to molecular motion