Chapter 4: Enzymes
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What are enzymes?
Enzymes are biological catalysts. The speed up the rate of reactions by lowering the activation energy of the reaction and are chemically unaltered at the end of the reactions, and thus can be reused, and they are effective in small amounts.
Explain the mode of action of enzymes [8]
Enzymes have an specific active site which is complementary in shape and charge to the substrate
Effective collision between enzyme and substrate form a temporary enzyme-substrate complex
Based on the lock and key hypothesis, enzyme is the lock and substrate is the key
ESC held together by weak interactions eg. H, I, H/phobic ions
Catalysis
Enzyme lowers the activation energy barrier by:
Aligning substrates next to each other in AS for rxn to occur
Distorts the substrate and reducing activation energy to achieve transition state
Orientates substrate such that bonds are exposed to attack
Provides a favourable micro environment
R-groups of amino acid residues in AS participate in direct catalysis (Eg. Acid-base catalysis)
Release
Products no longer fit active site and are released → enzyme is unchanged and can be used again
What are the 2 models that explain specificity of enzymes?
Lock and key hypothesis
Enzyme is lock and substrate is key
Enzyme active site has a conformation which is complementary in shape and charge to a specific substrate
When enzyme and substrate molecules collide in the correct orientation the substrate will bind to the active site and form an enzyme-substrate complex
Catalysis occurs
Products no longer fit AS → leave AS, allowing other substrate molc to bind to AS
Induced fit hypothesis
Binding of substrate to AS induces a conformational change in the AS such that it now provides a more precise fit for the substrate
Enzyme can perform its catalytic function more effectively
Amino acid residues on enzymes
Contact residues
Found in AS, helps to position substrate in the correct orientation via weak interactions eg…
Catalytic residues
Found in AS, have specific R groups which act on bonds in the substrate, help to catalyse the conversion of substrate to product
Structural residues
Interact with each other, maintain overall 3D conformation of protein
Non- essential residues
Found on surface of protein, no specific function
How do enzymes lower activation energy?
Proximity effects
Catalysed: Reactants next to each other temporarily bind in AS → incr chances of reaction
Vs Uncatalysed: depend on random collisions
Strain effects
Reactants slightly distort as they bind to enzyme → strain bonds which are to be broken → incr chance of breakage
Orientation effects
Enzyme holds reactants so that bonds are exposed to chemical attack by catalytic R groups
Microenvironment effects
Hydrophobic AA create water-free zone → non-polar reactants react more easily
Acid-base catalysis
Acidic and basic AA in enzymes facilitate catalysis
Effect of desaturation on enzyme active site
Denaturation results when H bonds, ionic bonds and other weak interactions that stabilize 3D conformation are broken
Conformation of enzyme is altered including that of AS
AS no longer complementary in shape and charge to substrate
Enzyme cofactors
Inorganic ions
Metal ions that change non-functioning AS into functioning one
eg. Mg ions in PCR
Mould enzyme or substrate and allows ESC to form more easily
Coenzymes
Eg. NAD transfers electrons in certain redox runs in respiration
Organic molcs required by certain enzymes to carry out catalysis
Bind to AS of enzyme and participate in catalysis but are not considered substrates of the rxn
Function as intermediate carriers of electrons or specific atoms that are transferred in the overall rxn
Prosthetic group
Eg. Haem group of cytochrome oxidase in ETC in inner mitochondrial membrane accepts electrons from cytochrome C and transfers them to oxygen to form water
Permanently bound to enzyme
transfers atoms/ chemical groups between AS of enzyme and another substance
Effect of temperature on enzyme-catalysed reactions
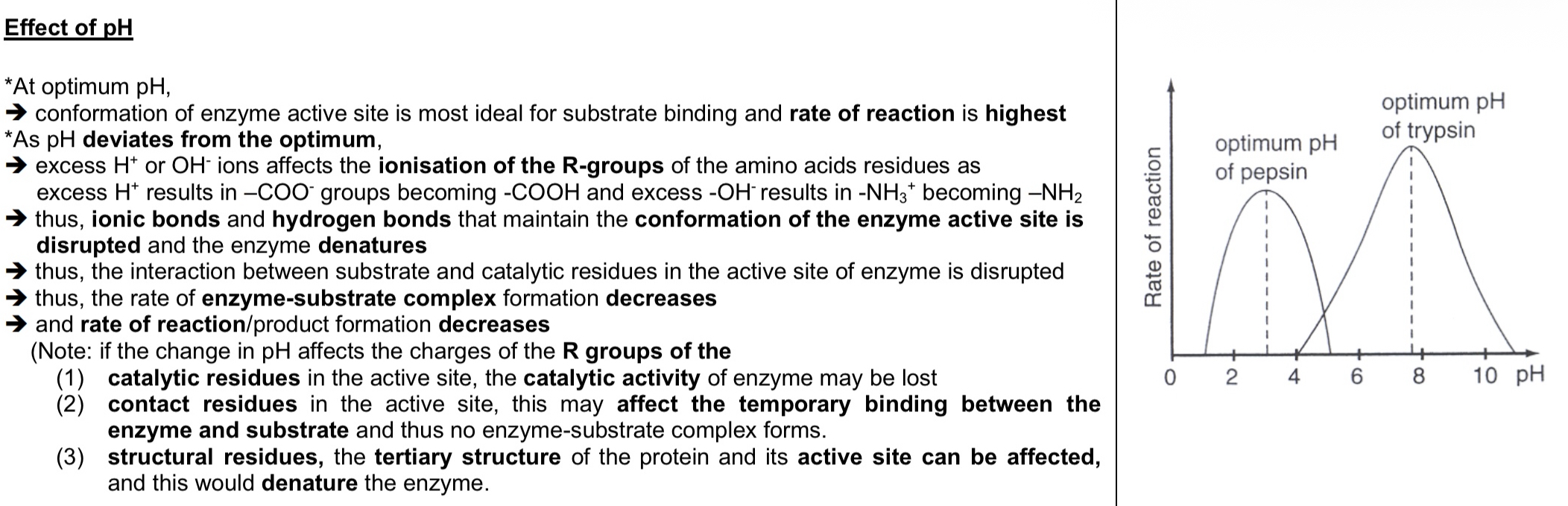
Effect of pH on enzyme-catalysed reactions
Effect on enzyme concentration on enzyme-catalysed reactions

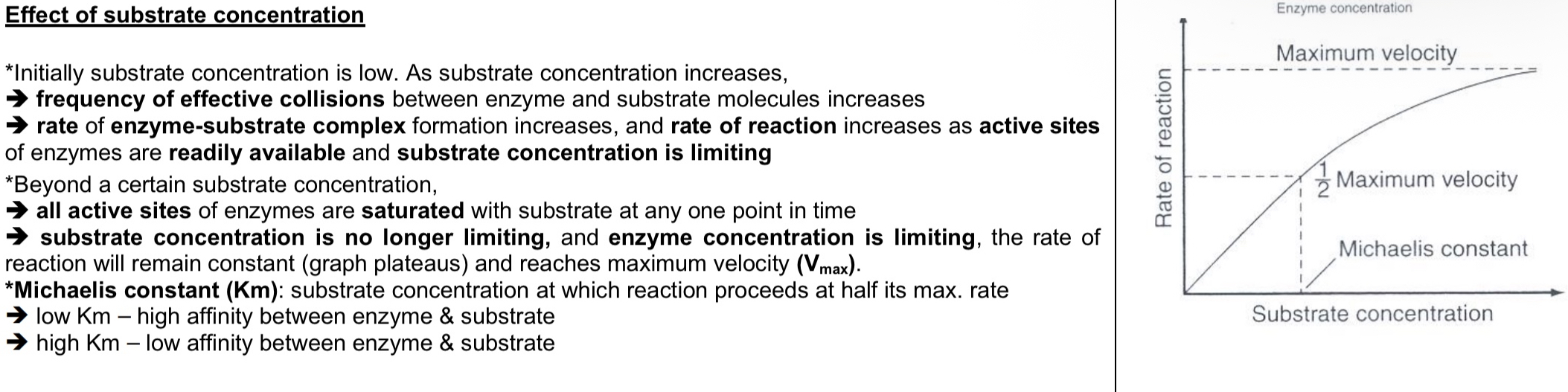
Effect of substrate concentration on enzyme-catalysed reactions