DDS Lecture 4 Content
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
Need for Various Dosage Forms
to protect drugs from degradation due to humidity or oxygen
to prevent chemical/enzymatic degradation of drugs in GI
to make the medicine palatable by masking unpleasant taste or odor
to provide optimal drug therapy by topical administration
to provide optimal drug therapy in the form of suppositories and inserts
to introduce drugs directly into circulation
for emergencies
to provide optimal drug therapy through inhalation therapy
nasal passages/airways
to provide liquid dosage forms for ease of administration
for very young , very old patients
General Considerations in Dosage form Design
nature of illness
treatment method (local vs systemic)
age and anticipated condition of patient
When are liquid dosage forms preferred?
in infants and children younger than 5
could choke, spit out tablets
patients with difficulty swallowing solid dosage forms
Types of liquid dosage forms
Solutions (uniform system)
Syrups
Elixirs
Tinctures
Dispersed Systems (2 distinct phases, most medications)
Emulsions
Suspensions
Preformulation Studies
basic study of physical and chemical characteristics of a drug substance before formulating the drug into a proper dosage form
employ knowledge gained through experience with structurally similar drugs
collaborative effort of physical, chemical, biological, pharamceutical sciences
Preformulation of Liquid Dosage Forms
Active Pharmaceutical Ingredient (API)
Chemical Properties
Physical properties
Stability
Excipients
Chemical properties considered in pre-formulation
structure
reactivity
form
Physical properties considered in performulation
physical description
particle size
crystalline structure
melting point
solubility
microscopic examination
heat of vaporization
melting point depression
particle size
polymorphism
dissolution
membrane permeability
partition coefficient
pKa/dissociation constants
Microscopic Examination
physical characteristic
provides information about particle size range and crystalline structure of drug
Heat of vaporization
amount of heat required to vaporize 1 g of liquid
important in volatile drugs
helps minimize exposure of personnel to hazardous drug vapors
physical characteristic
Melting point depression
pure compounds have defined melting points
impurities can chance (decrease) melting point
gives information about purity and compatibility with other compounds
physical characteristic
Particle size
physical characteristic
size and distribution affects dissolution rate, bioavailability, content uniformity, texture, taste
sedimentation rate in liquid dosage forms depends highly on particle size and equal distribution of API throughout formulation
affects suspendibility in liquid vehicle
affects texture in ophthalmic preparations and parenterals
Polymorphism
physical characteristic
1/3 organic compounds exist in multiple crystalline form
polymorphic forms have different melting points, solubility, chemical and physical stability
Solubility
affected by particle size and pH
decrease in particle size increases the surface area and solubility
solubility of weak acids and weak bases are pH dependent
Water Soluble inorganic molecules
ionic compounds containing monovalent cation and anion
common salts of alkali metals (Na, K)
Quaternary ammonium salts
nitrates, nitrites, acetates, chlorates
sulfates and sulfites (EXCEPT Ca and Ba sulfates)
Chlorides, bromides, iodides (EXCEPT silver salts)
Water insoluble inorganic molecules
hydroxides and oxides of non-alkali metals (EXCEPT alkali metals and ammonium ions)
sulfides (EXCEPT alkali metal salts)
Phosphates, carbonates, silicates, borates, hypochlorites (EXCEPT: alkali metal salts, ammonium salts)
General Rules of Solubility for Organic Molecules
most drug molecules are organic
one polar functional group can solubilize 5 carbonds
molecules having branched chains are more soluble than straight chains
water solubility decreases with increases in molecular weight
structural similarity with solute and solvent = increased solubility
Very soluble
<1 parts solvent for 1 part solute
Freely soluble
1-10 parts solvent required for 1 part solute
Soluble
10-30 parts solvent required for 1 part solute
Sparingly soluble
30-100 parts solvent for 1 part solute`
Slightly Soluble
100-1000 parts solvent for 1 part solute
Very Slightly Soluble
1000-10,000 parts solvent for 1 part solute
Practically insoluble or insoluble
>10,000 parts solvent for 1 part solute
Membrane permeability
physical characteristic
depends on pKa, solubility, dissolution rate, lipid solubility of drug molecule
evaluated in pre-formulation by studying drug transport rate in everted intestinal sac model
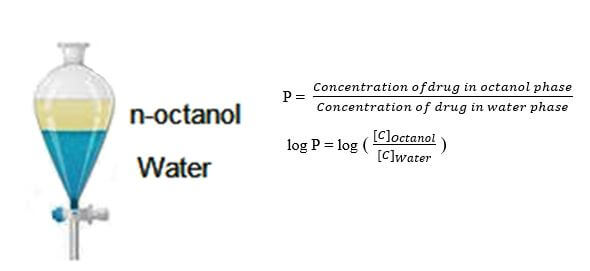
Partition Coefficient
physical characteristic
measure of molecule’s lipophilic character
lower log P = hydrophilic
higher log p = lipophilic
ideal range: positive, less than 5
octanol chosen for formula because 8C chain mimics lipid bilayer
pKa/Dissociation constant
physical characteristic
degree of ionization is dependent on pH
degree of ionization affects solubility, absorption, distribution, elimination
pKa important when forming admixtures of drugs
should not mix drugs with very different pKa, can cause precipitation
Physical Stability
can see without testing
not always permanent
Chemical stability
cannot see without testing
can sometimes be smelled
can be permanent
Most common drug instability
chemical degradation by hydrolysis or oxidation
Hydrolysis
affects esters, amides, lactones, lactams
drug molecules interact with water molecules to yield breakdown products
ex. aspirin + water → salicylic acid + acetic acid
if it smells like vinegar, aspirin has degraded by hydrolysis
Oxidation
affects alcohols, aldehydes, alkaloids, phenols, sugars, unsaturated fats and oils
loss of electron
many are auto-oxidation: occur spontaneously under the initial influence of atmospheric oxygen and proceed slowly at first and then more rapidly
triggered by light exposure
Protection against hydrolysis
using water substitute (glycerin, propylene glycol, alcohol)
vegetable oils used for some injections
making suspension in non-aqueous vehicle than making an aqueous solution
making drug formulation in dry form ready for reconstitution in pure water prior to use
most commonly used
refrigerating
prevent microbial growth
reduce temperature catalyzed hydrolysis
maintain pH between 5-6
hydrolysis more stable at acidic pH
Protection against oxidation
including antioxidants in formulation
replacing oxygen with nitrogen in formulation bottles
chelating trace metals in the formulation
packaging in light resistant containers
store in cool, dark place
Sodium sulfite
antioxidant for high pH
Sodium bisulfite
antioxidant for intermediate pH
sodium metabisulfite
antioxidant for low pH
Common excipients in liquid formulations
flavoring agents
sweetening agents
coloring agents
preservatives
Electronic Tongue
provides information on bitterness levels and the stability of flavors in terms of taste
“taste fingerprint”
Flavoring Agents
excipients
used for imparting new flavor or masking undesirable flavor
3 types: natural, artificial, spice
added to liquid formulations to mask bad taste
color, odor, texture, and taste should match
milder flavors used for long-term medications
to avoid flavor fatigue
not recommended for infants under 3-6 months
Natural AA flavor
all components derived from AA
exact composition not known
AA flavor - natural and artificial
at least one component derived from AA
no definition of natural-to-artififcial ratio
AA flavor with other natural flavors (WONF)
all components are natural
at least one component is derived from AA
Natural flavor-AA type
all components are natural
no component is derived from AA
AA flavor- artificial
all components are artificial
Conceptual flavor
may contain artificial flavors
no reference point
may only have to declare in ingredient declaration
Suggested flavors to mask salty tastes
cinnamon, raspberry, orange, maple, butterscotch, licorice
Suggested flavor to mask sweet taste
fruit, berry, vanilla
suggested flavor to mask bitter taste
cocoa, chocolate, mint, cherry, walnut, licorice, raspberry
suggested flavor to mask sour/acid taste
fruit, citrus, cherry
suggested flavor to mask oily taste
wintergreen, peppermint oil, lemon, anise
suggested flavor to mask metallic taste
mint, marshmallow
Flavors to mask antibiotics
cherry
maple
pineapple
orange
strawberry
vanilla
banana-pineapple
Flavors to mask antihistamines
apricot
cherry
cinnamon
grape
honey-lime
peach-orange
Flavors to mask barbiturates
banana-pineapple
banana-vanilla
cinnamon-peppermint
lime
grenadine strawberry
Flavors to mask decongestants
apricot
cherry
buterscotch
strawberry
lemon
maple
orange
tangerine
coriander
Flavors to mask Electrolyte and geriatric solutions
cherry
lemon-lime
grape
strawberry
lime
raspberry
root beer
Sweetening Agents
colorless and odorless
soluble in water
pleasant tasting
free from after taste
stable over wide pH range
Sweetner examples
acesulfame potassium (200x sweeter than sucrose)
aspartame (200x sweeter than sucrose)
sodium saccharin (600x sweeter than sucrose)
saccharin (300x sweeter than sucrose)
Coloring Agents
impart color
contraindicated in sterile solutions
dark colors generally not used (dark purple, navy, black, brown)
natural colors (red ferric oxide, titanium oxides) and FDA approved synthetic dyes (D&C and FD&C)
Preservatives
Should…
prevent microbial growth
prevent growth of most likely contaminants
soluble in aqueous phase of preparation in adequate concentration
percentage of undissociated preservative at pH should be capable of permeating microbial cell wall
non-irritating, non-sensitizing, non-toxic
chemically stable during shelf-life
compatible with other ingredients
Mechanisms of Preservatives
partial lysis of cell wall
lysis and cytoplasmic leakage
protein denaturation
inhibition of cell wall synthesis or enzyme systems
oxidation/hydrolysis of cellular contents
Preservatives that partially lyse cell wall
phenols
alcohols
quaternary compounds
Preservatives that act by lysis and cytoplasmic leakage
phenols
alcohols
quaternary compounds
Preservatives that act by protein denaturation
benzoic acid
alcohols
boric acids
p-hydroxybenzoates
Preservatives that act by inhibition of cell wall synthesis or enzyme systems
mercurials
General Formulation Considerations
formulation prototypes developed after pre-formulation evaluation of API
liquid dosage form primarily consists of API, solvent/diluent, co-solvents, preservatives
oral liquid dosage forms may have coloring and flavoring agents in addition to sweetening agents
viscosity of product is important for palatability and suspending properties
also for ease of pour
Types of liquid dosage forms
oral
parenteral
topical
other
Oral liquid dosage forms
solutions
suspensions
emulsions
liquid-filled soft and hard gel capsules
Parenteral liquid dosage forms
solutions
suspensions
emulsions
Topical liquid dosage forms
solutions
suspensions
emulsions
Other liquid dosage forms
otic products
nasal sprays
ophthalmic
Solubility
important to determine if formulation will stay in solution for life of product
affected by temperature, electrolytes, complexation with other components
solubility studies carried out at formulation stage
solubility can be increased by salt formation
Viscosity
measure of resistance of fluid deformed by either sheer stress or tensile stress
viscosity will affect flow properties and dispensing
viscosity enhancers can be used to allow dosing control
Packaging liquid dosage forms
viscosity and dosage form determines type of container
high temperature enhances flow properties
in most cases, pump system utilized to deliver product to container through filter (sterile or non-sterile)
Process Validation
identifies critical steps in manufacturing process
limits specified for mixing times, heating ranges, room conditions
effect of above factors on incorporation of API, preservatives, excipients
Stability (USP)
extent to which a product retains, within specified limits, and throughout its period of storage and use (shelf life), the same properties and characteristics that it possessed at the time of manufacture
Shelf-life
time for original potency of the API to be reduced to 90%
Kinetics and Shelf-life
chemical stability affected by temperature, light, humidity
chemical stability of API determines shelf-life of dosage forms
kinetics = just drug
shelf life = whole formulation stability
stability and expiration date based on kinetics of degradation reactions
zero-order rate reactions
first-order rate reactions
Shelf-life estimation
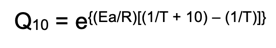
Kinetic studies
measure concentration of drug at given intervals under specific pH, temperature, ionic strength, light intensity, and drug concentration
Importance of Kinetics
selection of proper storage temperature
selection of proper container for dispensing
anticipation of interactions when mixing drugs and dosage forms
dissolution determinations
Estimating shelf life at given temperatures

increase in change in temperature will decrease shelf-life
decrease in change in temperature will increase shelf-life
Average, best estimate for Q10 = 3
lower limit = 2
upper limit = 4
Drug X has a shelf life 240 hours when stored in a refrigerator (50C). The estimated shelf life of Drug X at room temperature (250C) will be ___________
( t90(T2) = t90(T1)/Q10(ΔT/10))
t90(T2) = 240/320/10
= 26.66 ~ 27 hours
What Stability testing considers
before approval and marketing, stability must be assessed
influence of pharmaceutical ingredients
influence of container and closure
manufacturing processes
packaging components
conditions of storage
anticipated conditions of shipping, temperature, light, humidity
anticipated durations and conditions of pharmacy shelf-life and patient use
Stability Testing
accelerated (6 months at 400C and 75% RH)
long term stability under usual conditions of transport and storage
sometimes samples are maintained at the long-term stability conditions for up to 5 years (250C ± 20C and RH of 60% ±5%)
gives information on drug product stability and actual shelf-life
Kinetic data tells you
reaction order
reaction rate
reaction order obtained experimentally by measuring reaction rate as function of concentration of degrading drug
chemical stability determined by reaction order
Factors affecting reaction kinetics
temperature
dielectric constant
ionic strength
solvent effect
catalysis
light
Physical paths of instability
polymorphs
cocoa butter, cortisone acetate
crystallization
solution, suspension
vaporization
flavoring agents, co-solvents
nitroglycerin
particle sedimentation
suspensions
Tablet visible signs of instability
appearance (cracking, chipping, mottling)
friability
hardness
color
odor
moisture content
clumping
disintegration
dissolution
Visible signs of capsule instability
moisture
tackiness
color
appearance
shape
brittleness
dissolution
Visible signs of powder/granule instability
appearance
color
odor
moisture
Visible Signs of Instability Coated tablets
integrity of coating
chipping
appearance (cracking, chipping, mottling)
friability
hardness
color
odor
moisture
content
clumping
disintegration
dissolution
Kinetics vs Stability
Kinetics:
Many half-lives
Pure systems (pure drugs)
to understand reaction mechanisms
Stability
85% drug remaining at endpoint
involves entire dosage form
purpose: establish expiration date
Drug Stability Assessment
chemically analyzed by HLPC with UV detection
HPLC: mobile phase, column, detector, pump, auto-sampler, data processing software (or integrator)
USP Stability Guidelines for Extemporaneous Formulation in Absence of Stability Information
non-aqueous and solid formulations in which manufactured drug is API = no later than 25% of time remaining for product’s expiration or 6 months (whichever is earlier)
non-aqueous liquid and solid formulations containing USP or NF substance as API = BUD of 6 months
Water-containing formulations prepared from solid form = BUD not later than 14 days in storage at refrigerated conditions
Other preparations = BUD 30 days or end of therapy (whichever is earlier)