3.3.4.1 Mass Transport in Animals PART 1
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
haemoglobin (Hb)
protein molecules with quaternary structure (more than 1 polypeptide)
-4 polypeptides + haem groups - contain iron ions which oxygen bind to + are carried to respiring tissues
LOADING or ASSOCIATION of oxygen
-haemoglobin binds with oxygen - takes in
-takes place in lungs
UNLOADING or DISSOCIATION of oxygen
-haemoglobin releases oxygen
-takes place in tissues
affinity
tendency for molecule to bind with oxygen
varies depending on the conditions of haemoglobin
-partial pressure of oxygen
-partial pressure of carbon dioxide
-saturation of haemoglobin with oxygen
pO2
a measure of oxygen concentration
-the greater the concentration of dissolved oxygen, the higher the partial pressure of oxygen
-as partial pressure increases, the affinity of haemoglobin for oxygen increases
if HIGH pO2
-oxygen loads onto haemoglobin to form oxyhaemoglobin
-in alveoli: high oxygen concentration, high pO2, high affinity, oxygen loads
if LOW pO2
oxyhaemoglobin unloads oxygen
-in respiring tissue: use up oxygen, low oxygen concentration, low pO2, low affinity, oxygen unloads
oxygen dissociation curve
graph that shows the relationship between the saturation of haemoglobin with oxygen and partial pressure of oxygen
saturation - volume of oxygen in haemoglobin
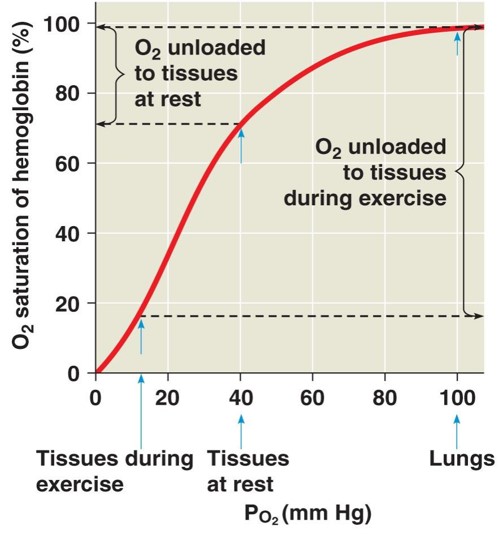
EXPLANATION of oxygen dissociation curve
1) shape of Hb makes it difficult for first oxygen to bind to haem group as polypeptides are close together - so low pO2 due to little oxygen binding so shallow graph
2) but binding of first oxygen changes quaternary shape of Hb so changes its shape
3) so second and third oxygen can bind to Hb easier as it takes smaller increase in pO2 so graph steepens = positive cooperative binding
4) however at fourth oxygen the majority of binding sites are occupied so less likely for O2 to bind so graph flattens
POSITION of oxygen dissociation curve
-the further to the left, the greater affinity of haemoglobin of oxygen (loads oxygen readily, unloads it less easily)
-the further to the right, the lower affinity of haemoglobin of oxygen (loads oxygen less readily, unloads it more easily)
carbon dioxide concentration
pCO2 - measure of concentration of CO2
-the Bohr effect = when cells respire, they produce CO2 so partial pressure of CO2 increases so Hb unloads O2 more as conditions become acidic so Hb changes shape and affinity for O2 decreases
how does respiration lower the pH of blood plasma?
-aerobic respiration produces carbon dioxide or carbonic acid which is acidic and releases H+ ions which lower the pH
-anaerobic respiration produces lactic acid which is also acidic
so due to higher rate of respiration haemoglobin have lower affinity for oxygen
DIFFERENT TYPES of haemoglobin
-low oxygen environments: organisms have haemoglobin with higher affinity for O2, due to little oxygen available needs to be good at loading any available O2
-high activity levels: organisms have haemoglobin with lower affinity for O2, needs to easily unload O2 so it is available to use
-size: small mammals = high SA:V ratio + have high metabolic rate so high O2 demand so have haemoglobin with lower affinity for O2 as needs to easily unload O2 to meet demand