Effects on Syntheses
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What are the three important effects that can become obstructions to go around when performing organic syntheses?
Steric effects.
Electronic effects.
Stereoelectronic effects.
Solvent and intermolecular effects.
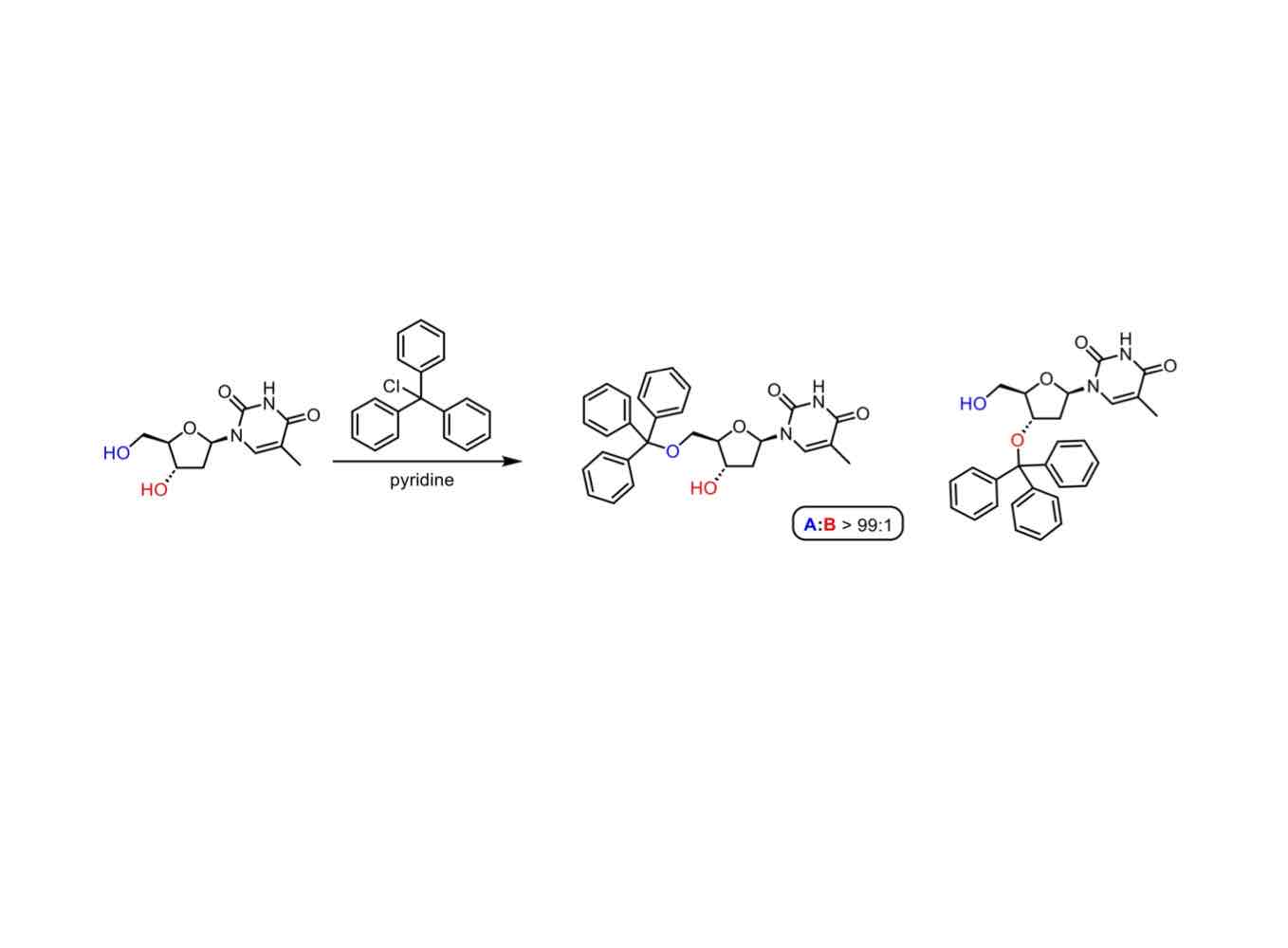
What are steric effects, actually?
Non-bonding interactions between substituents or reacting molecules, i.e. interaction of filled orbitals.
What are electronic effects, actually?
Distribution and movement of electrons within molecules, affecting their properties.
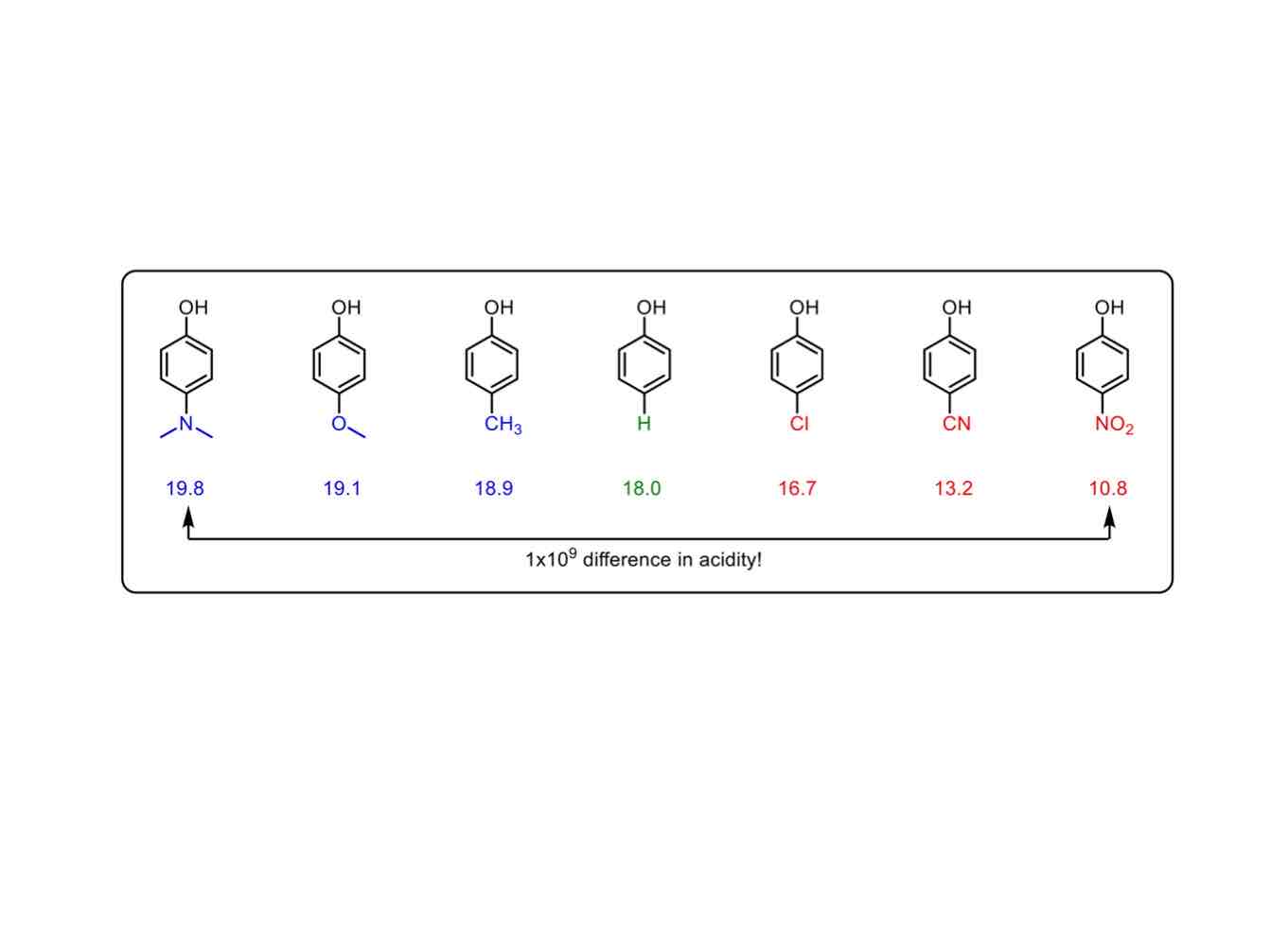
What do electronic effects primarily affect?
Electronic effects affect reaction rates and selectivities.
Anti-bonding orbitals are slightly MORE “anti-bonding” (i.e., destabilized relative to parent AOs) than bonding MOs are “bonding” (i.e., stabilized relative to parent AOs). Why?
Repulsion between two positively charged nuclei being brought together with no electrons in between to mitigate.
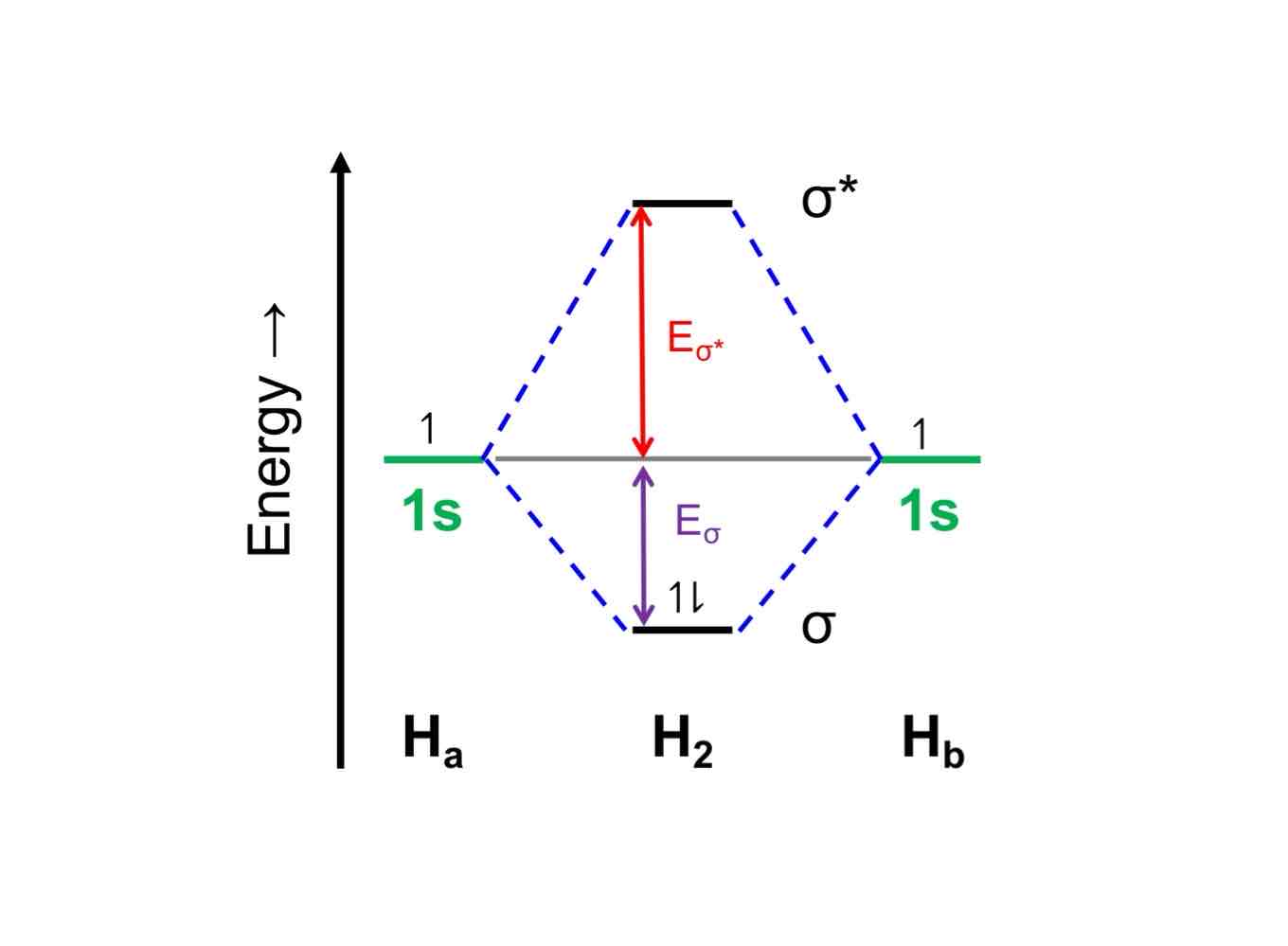
When it comes to molecular orbital diagrams, we arrange the orbitals, both molecular and parent atomic orbitals, by their energy. What is this "energy"?
What is the relationship between this energy and (a) stability and (b) distance from nucleus when the energy decreases and increases?
The energy that it takes to get an electron into their molecular orbitals.
Lower energy orbitals are more stable and closer to the nucleus, while higher energy orbitals are less stable and farther from the nucleus.
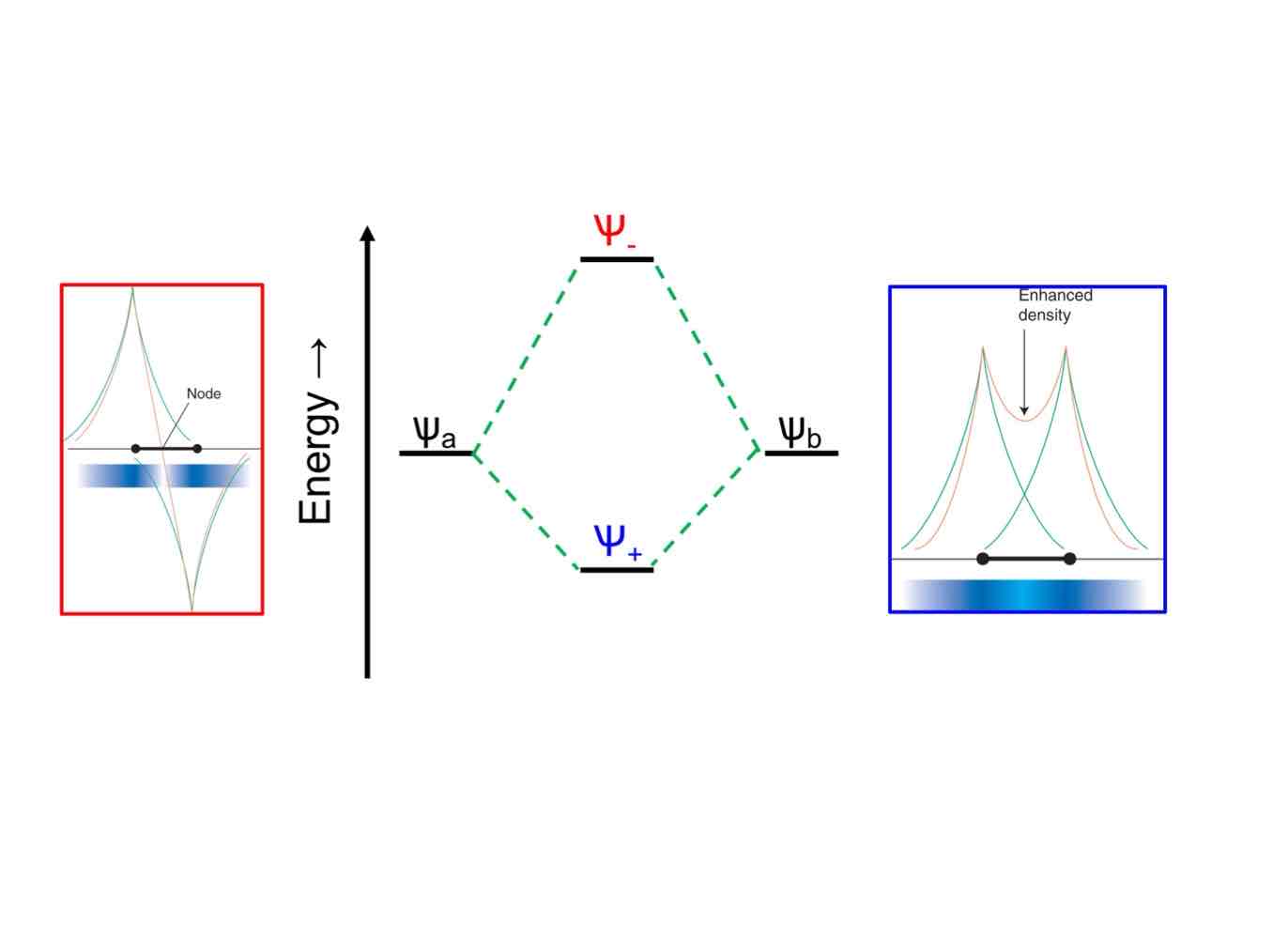
Why does energy increase away from the nucleus?
It takes more energy to distance an electron away from the nucleus because of Coulombic forces.
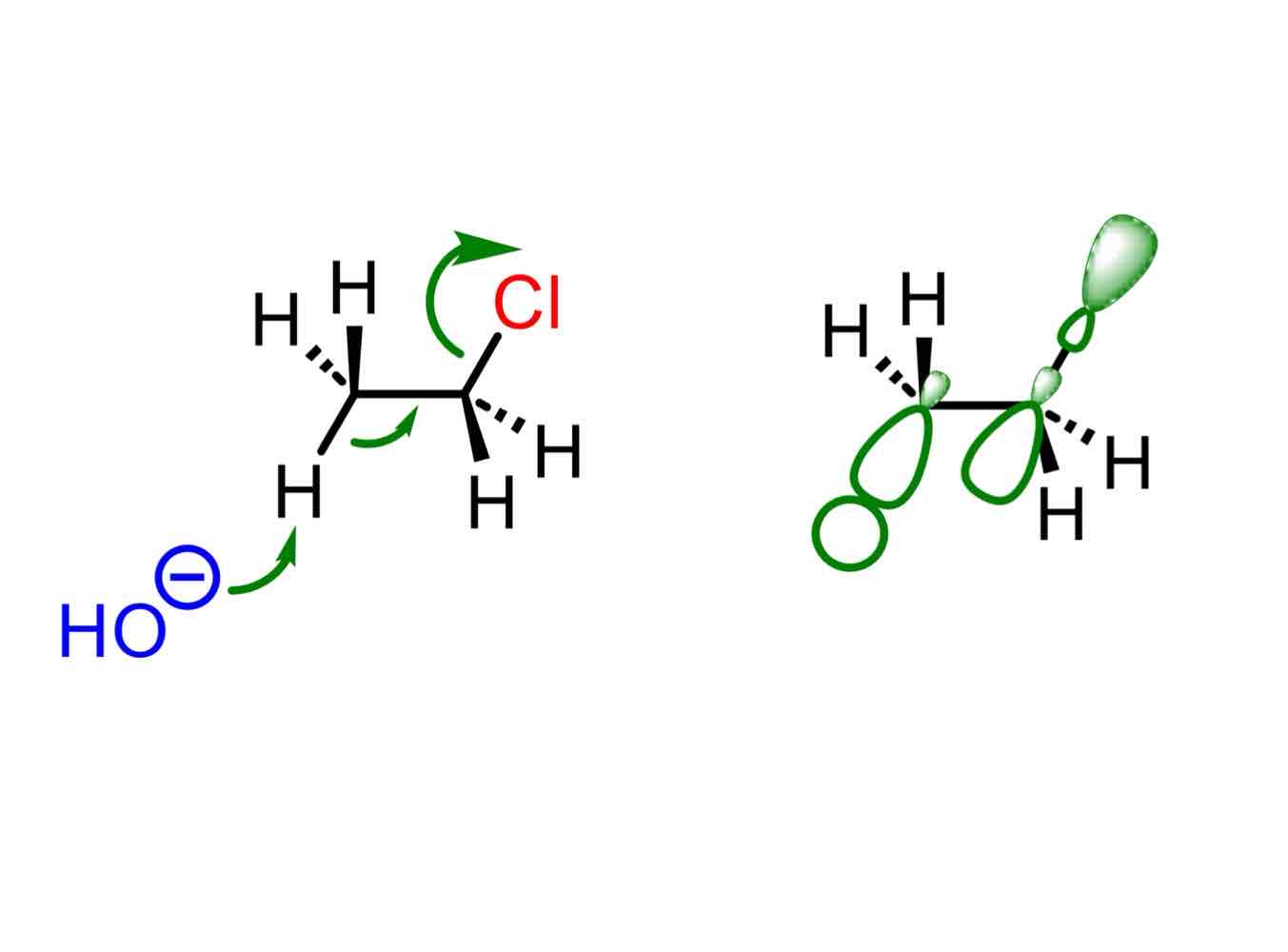
What are stereoelectronic interactions? What do they do? What maximizes these interactions?
Interactions between electrons that stabilize, maximized by a particular geometric arrangement which can be traced to favourable orbital overlap.
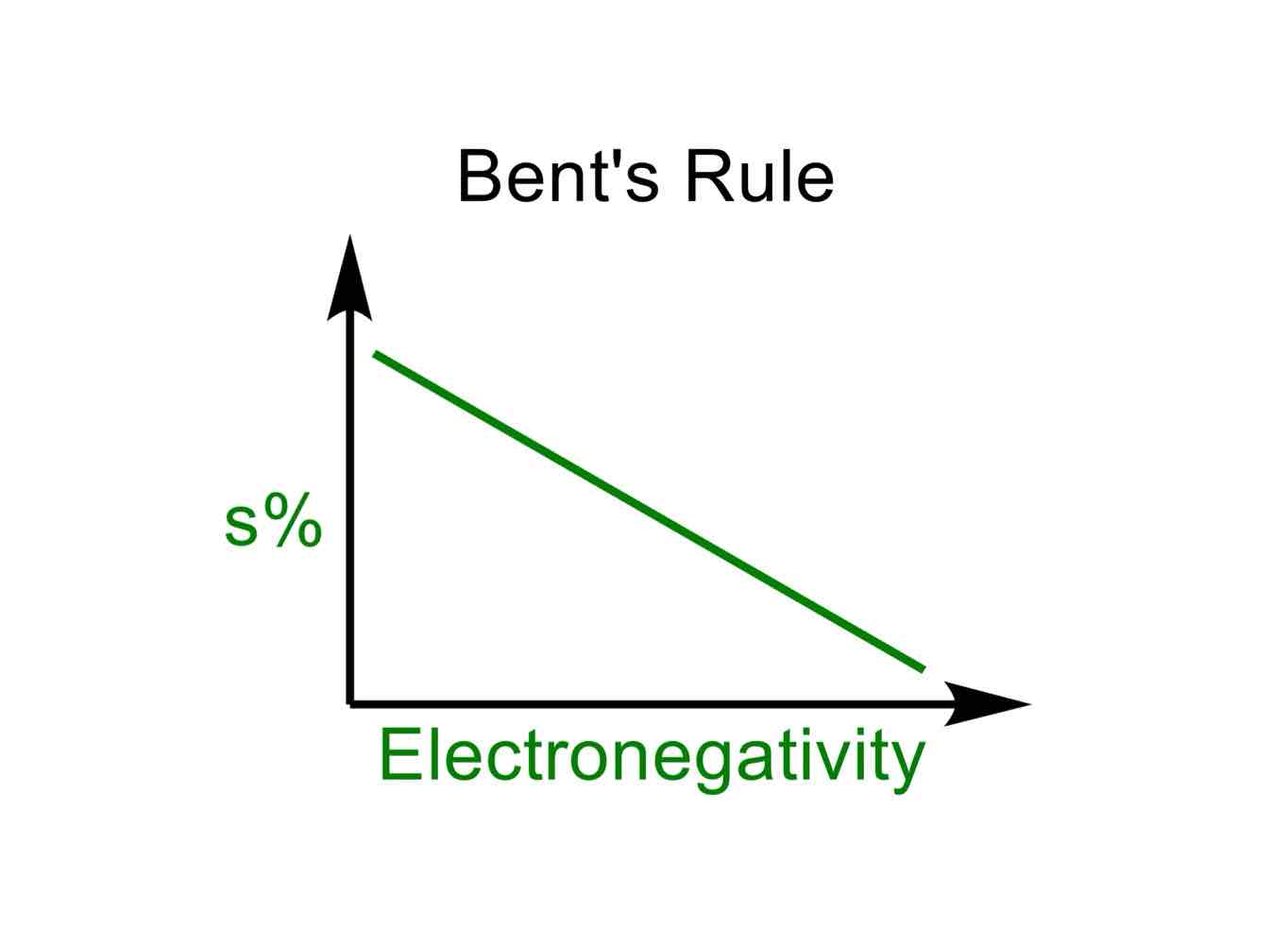
One thing to note about hybridization, however, is that there can exist unusual hybridized orbitals, like sp8.3, sp5, and the list goes on. In the context of electronegativity, why does this occur?
When a really electronegative atom is present, there will be more p-character in the hybrid set of orbitals on the atom that the electronegative atom is connected to, as there is more electron density there.
In other words, as EN of atom increases, less s-character in bond and more p-character. As EN of atom decreases, more s-character in bond and less p-character.
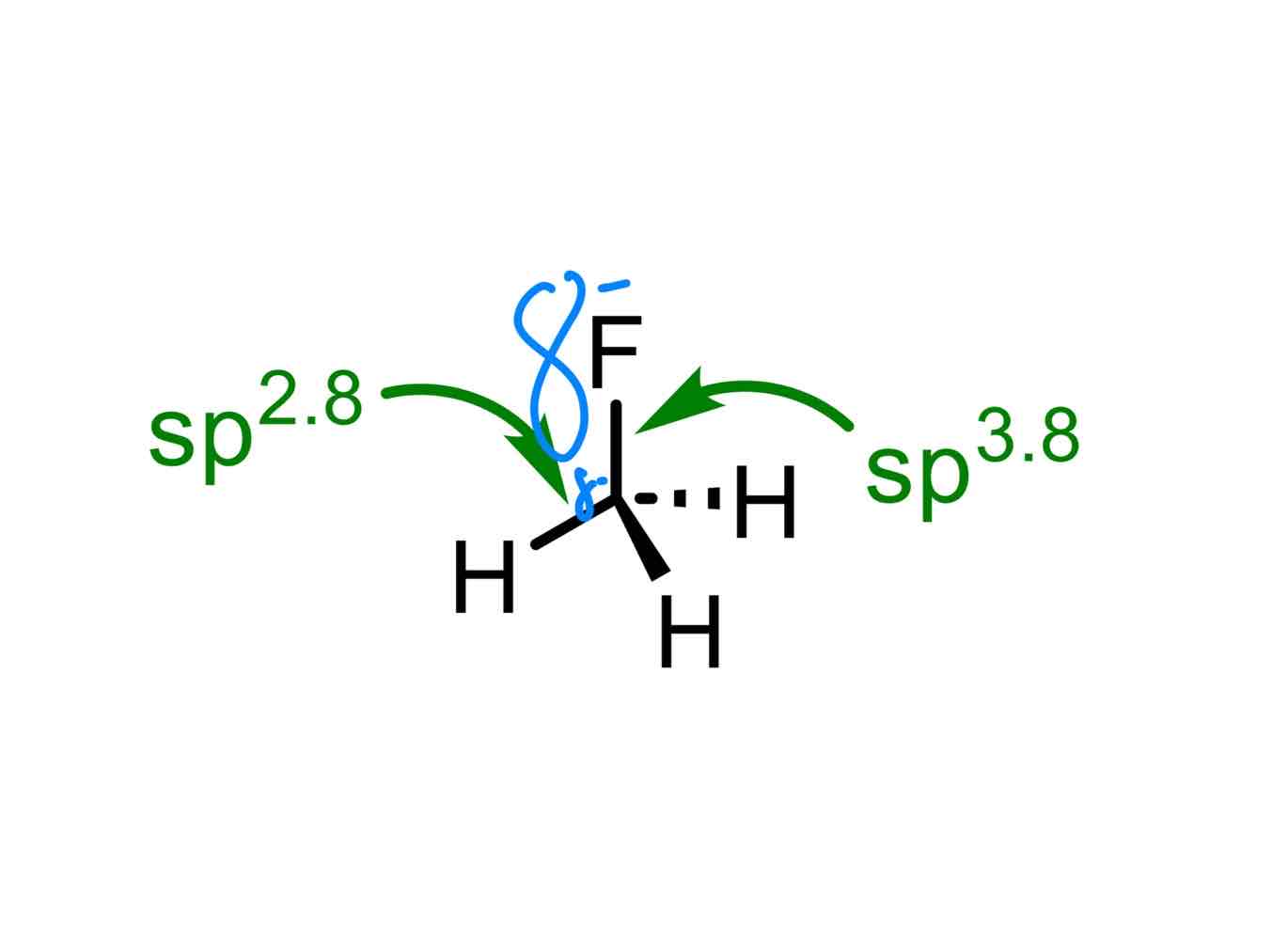
Why will there be more electron density in the p-character of the hybrid set of orbitals?
Let’s call the atom that the electronegative atom is connected to the “central atom.”
Electron density will end up in the p-character of the hybrid set because the s-orbital is closer to the centre of the central atom, while p-orbitals are farther out from the centre of the central atom. Because there is an electronegative atom present, this atom will take more of the electron density away from the s-orbital of the central atom and closer to itself.
How does s-character relate to the strength and stability of a bond?
More s-character, stronger bond.
How does angle strain affect p-character of a hybrid orbital?
Only know via computations.
Can the same atom have different hybrid orbital when connected to different atoms with different electronegativities?
Yes. Think of it as a seesaw. When one bond gains more p-character and less s-character, the other bond gets less p-character and more s-character.
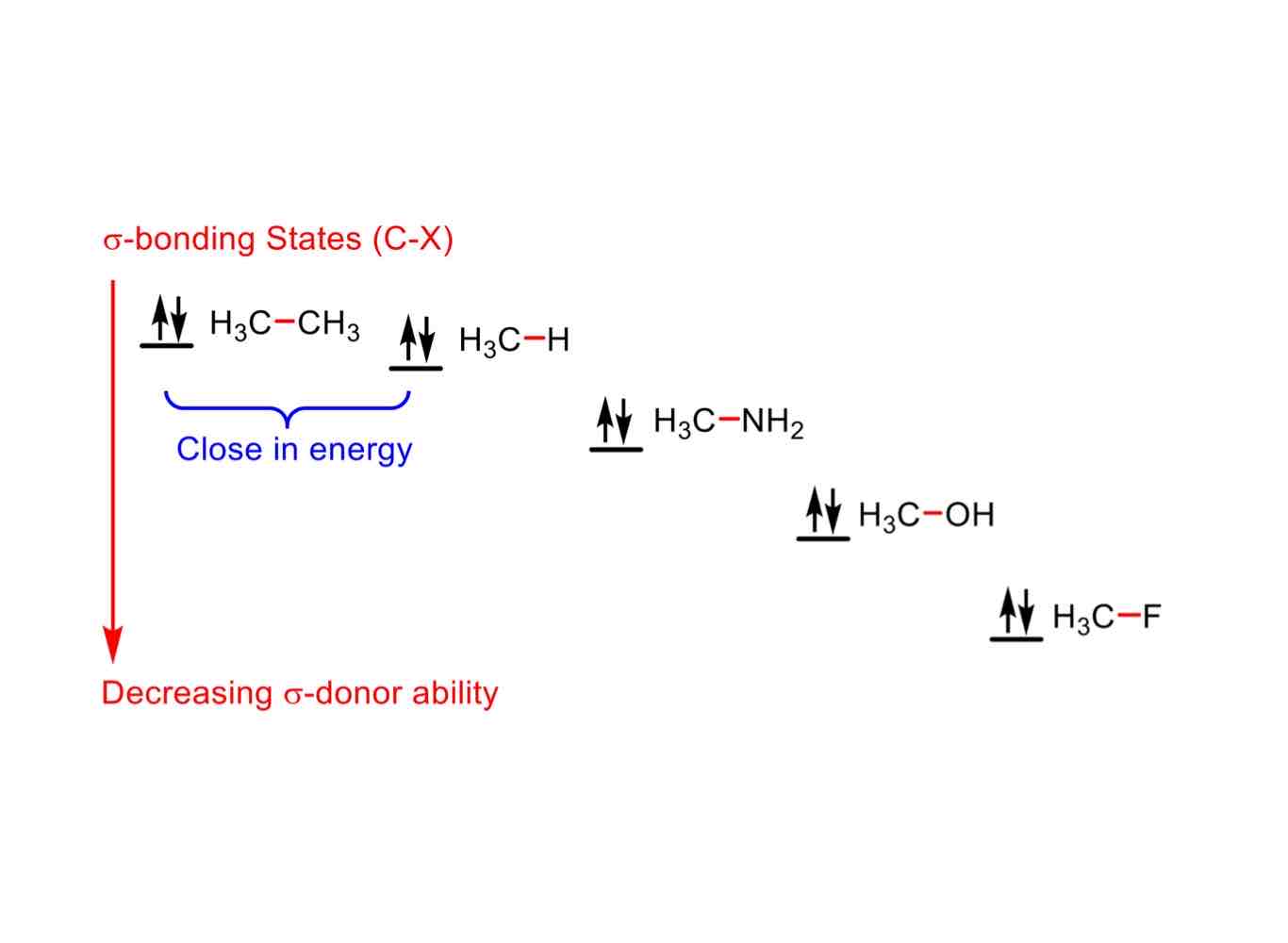
In its σ-bonding state, order these in increasing σ-donor ability when bonded to H3C:
CH3
H
NH2
OH
F
H3C-F (least donating)
H3C-OH
H3C-NH2
H3C-H
H3C-CH3(most donating)
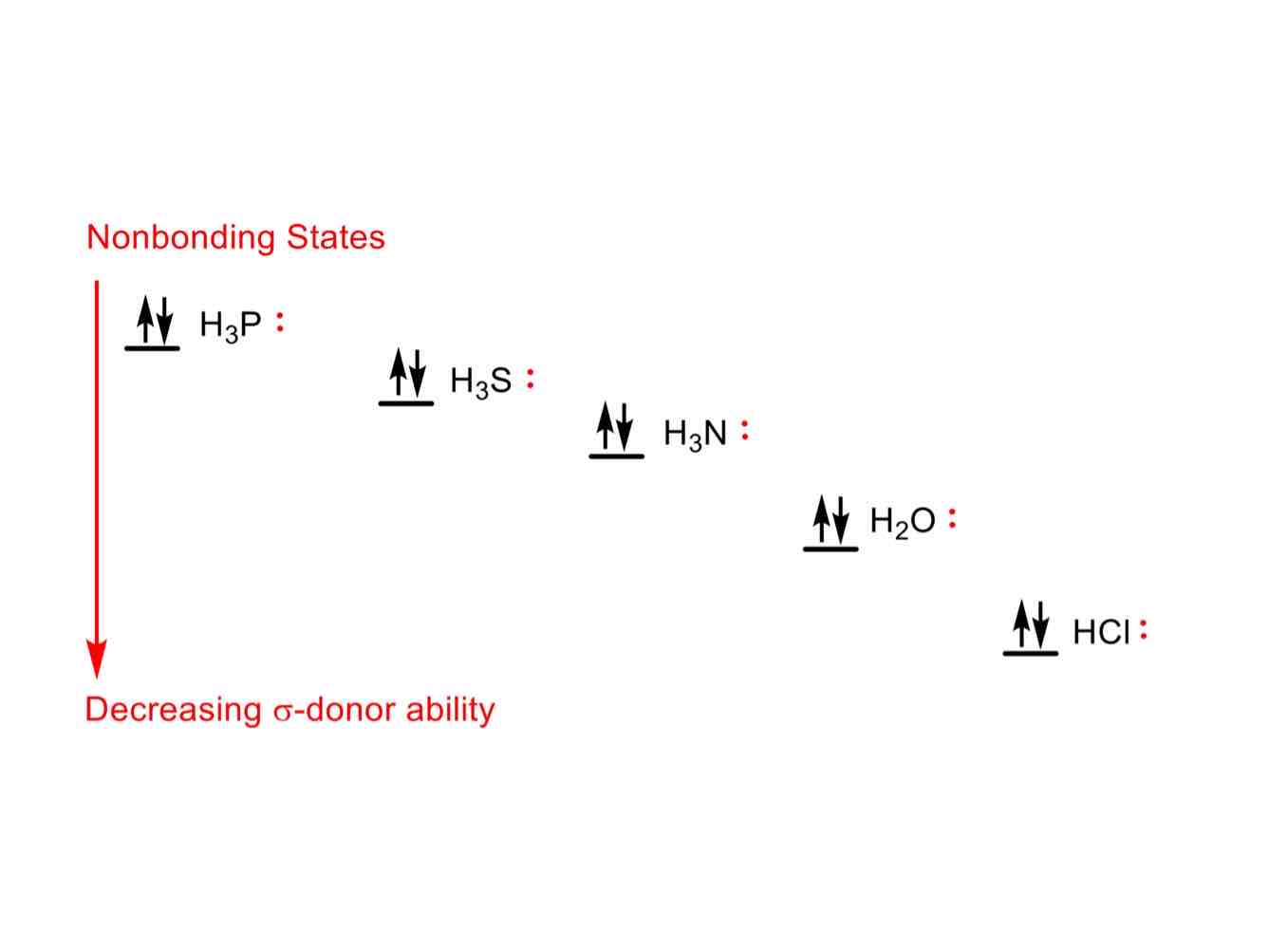
In its nonbonding state, order these in increasing σ-donor ability:
H3P
H3S
H3N
H2O
HCl
HCl (least donating)
H2O
H3N
H3S
H3P (most donating)
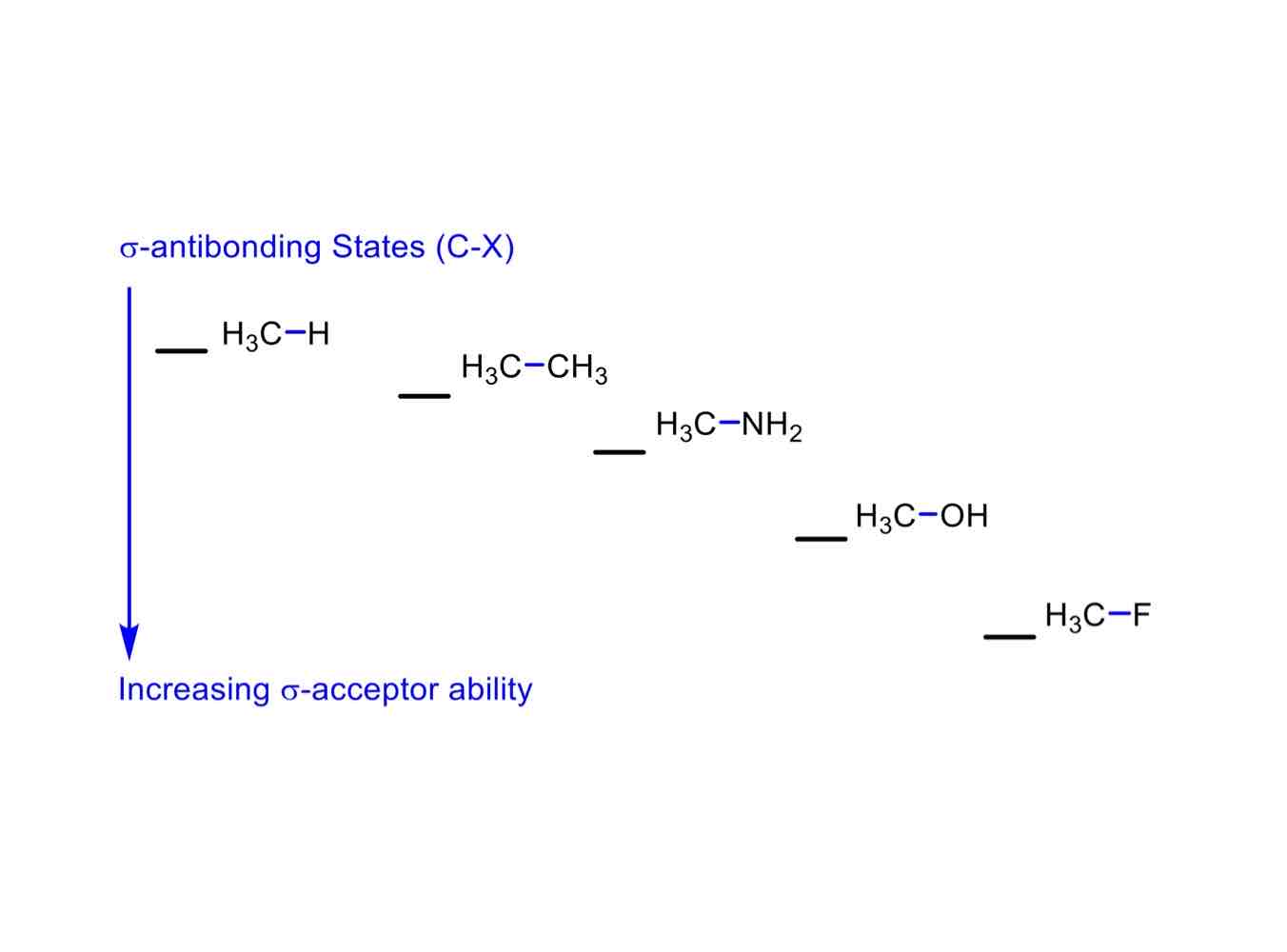
In its σ-antibonding state, order these in increasing σ-accepting ability when bonded to CH3:
CH3
H
NH2
OH
F
H3C-H (least accepting)
H3C-CH3
H3C-NH2
H3C-OH
H3C-F (most accepting)
It _______ energy to make a hybrid set of orbitals, but the energy is _______ by making sigma bonds.
It requires energy to make a hybrid set of orbitals, but the energy is lowered by making sigma bonds.
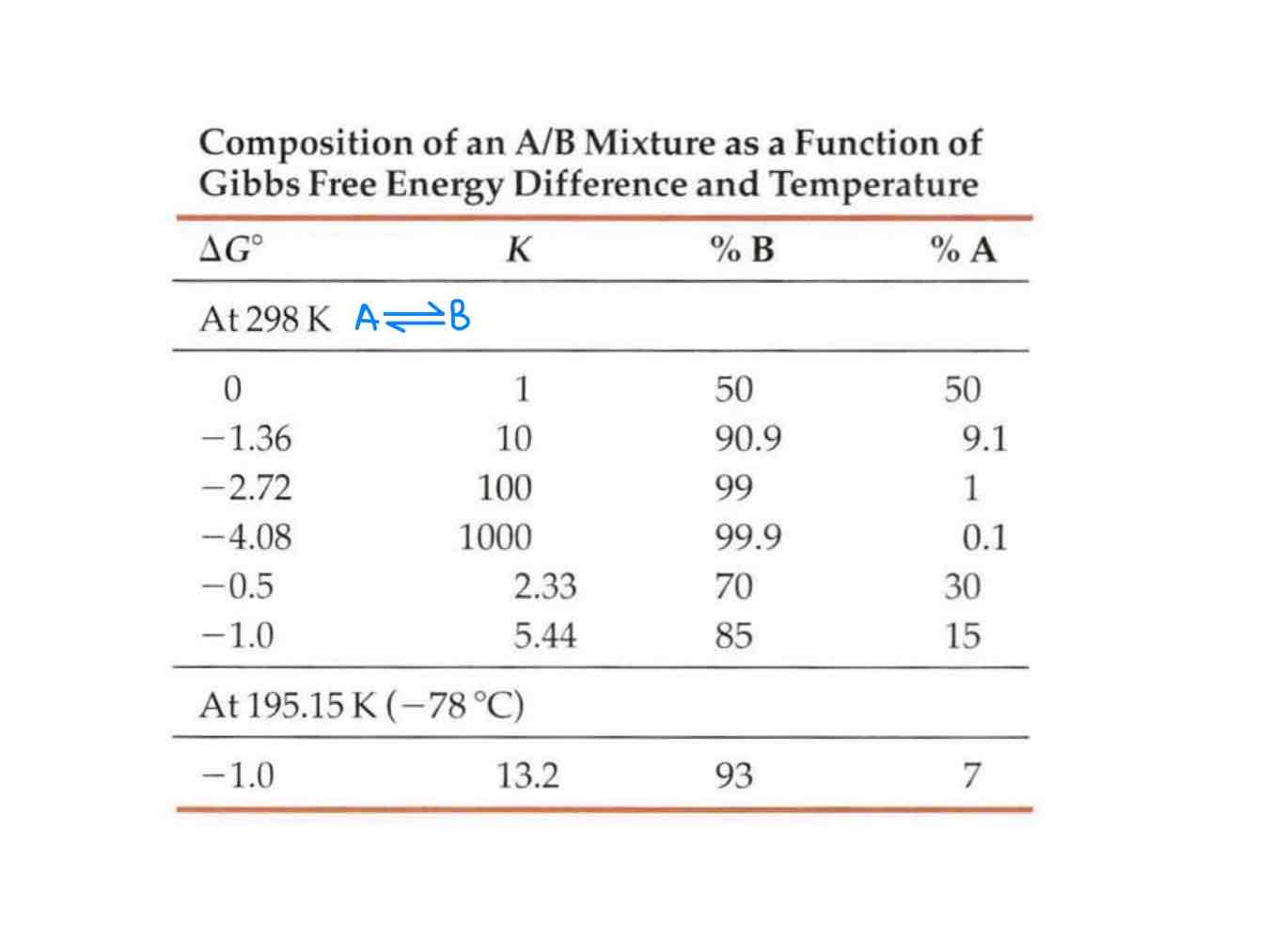
What does the rule of 1.4 state?
At 298K, if the ∆G changes by 1.4, depending on whether the 1.4 is negative or positive, making ∆G either negative or positive, there would be a ratio of of 1:10 or 10:1 respectively.
How come strain energy per carbon atom increases after having 6 carbons in a cyclic system?
Possibly multiple factors, but usually transannular effects - effects across the ring, e.g. hydrogen atoms not clearly visible or found.
What are the three general types of strain in saturated cyclic systems?
Angle (Baeyer) strain.
Torsional (Pitzer) strain.
van der Waals (steric) strain.
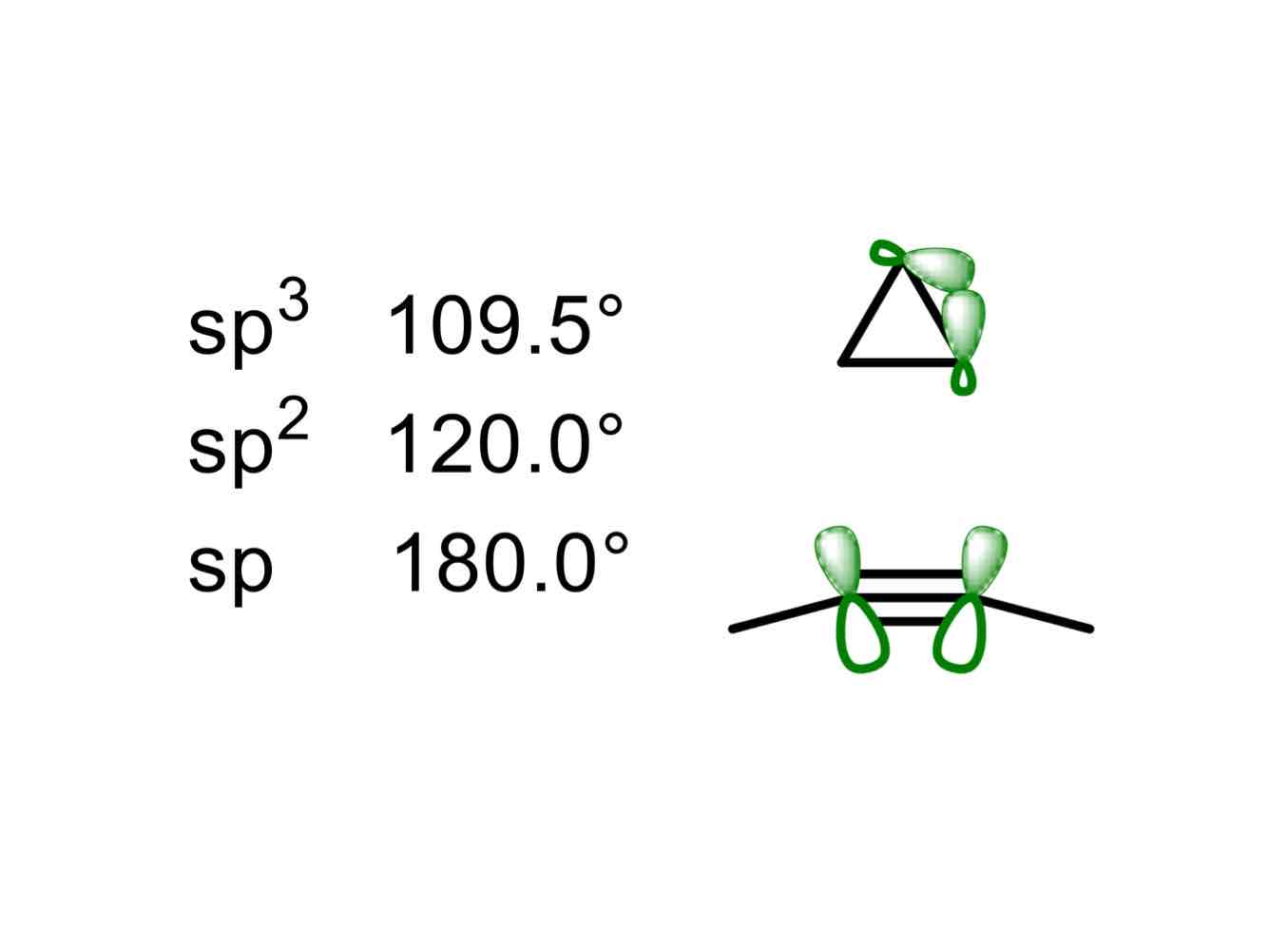
What causes Baeyer strain?
Strain arising from angular deviations from the measured and calculated optimal angle for an sp3 hybridized carbon atom, that of 109.5º.
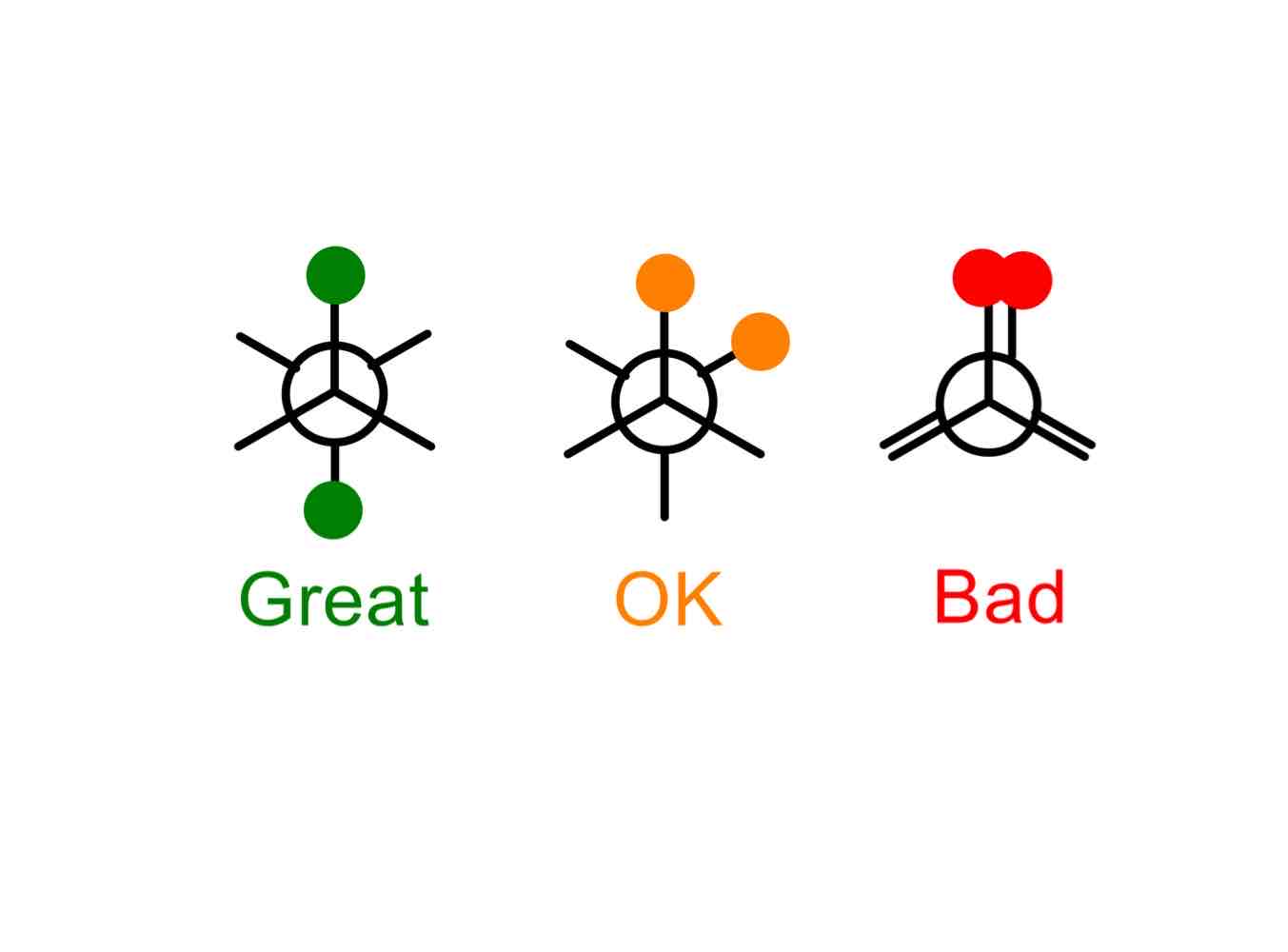
What causes Pitzer strain?
Strain arising from the stereoelectronic effect of a filled bonding orbital not being able to hyperconjugate with a nearby empty antibonding orbital
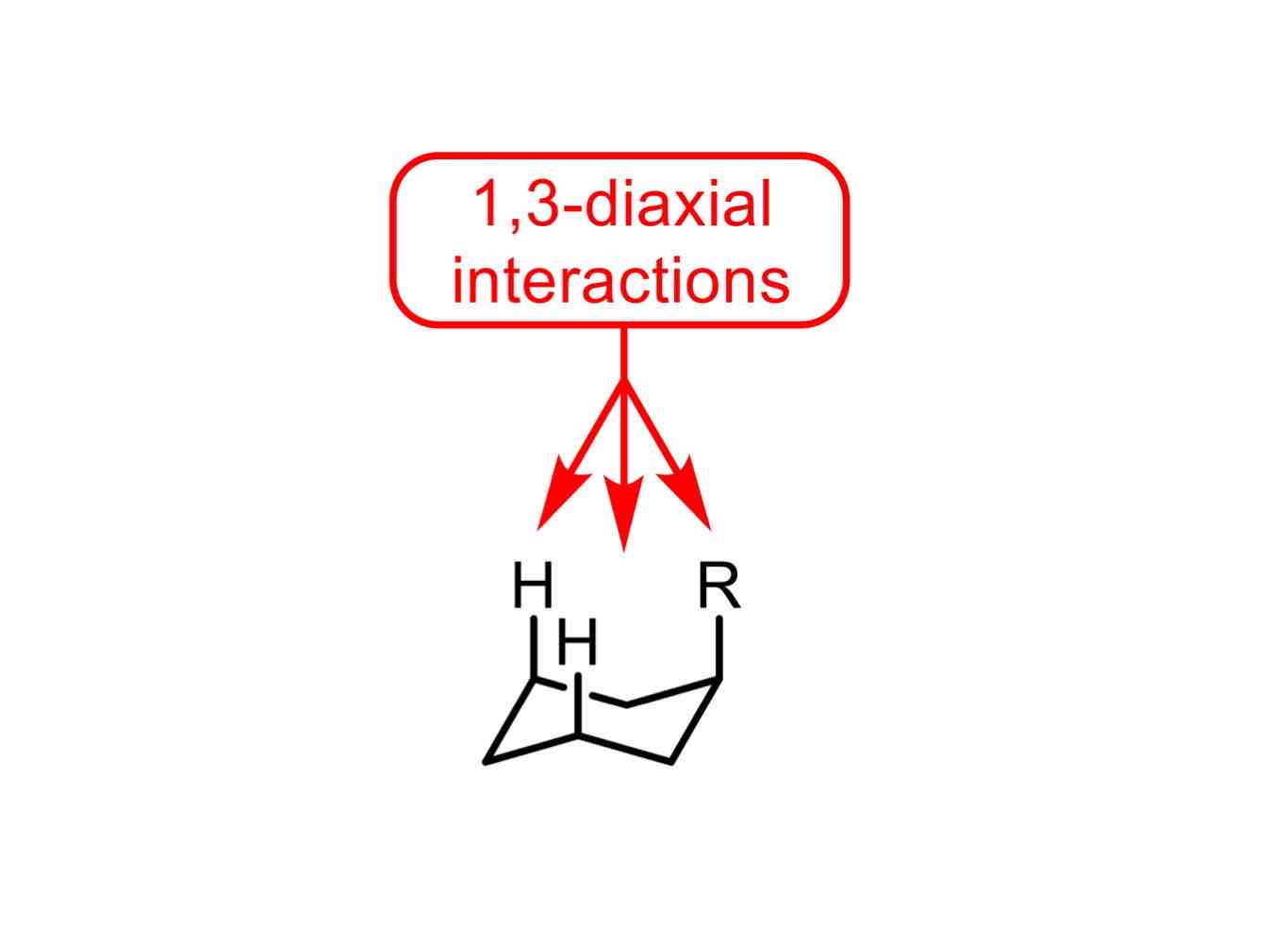
What are 1,3-diaxial interactions?
Steric interactions wherein “1,3” describes the distance between a substituent in the “1” position on a cyclic molecule and each of the atoms in the “3” positions.
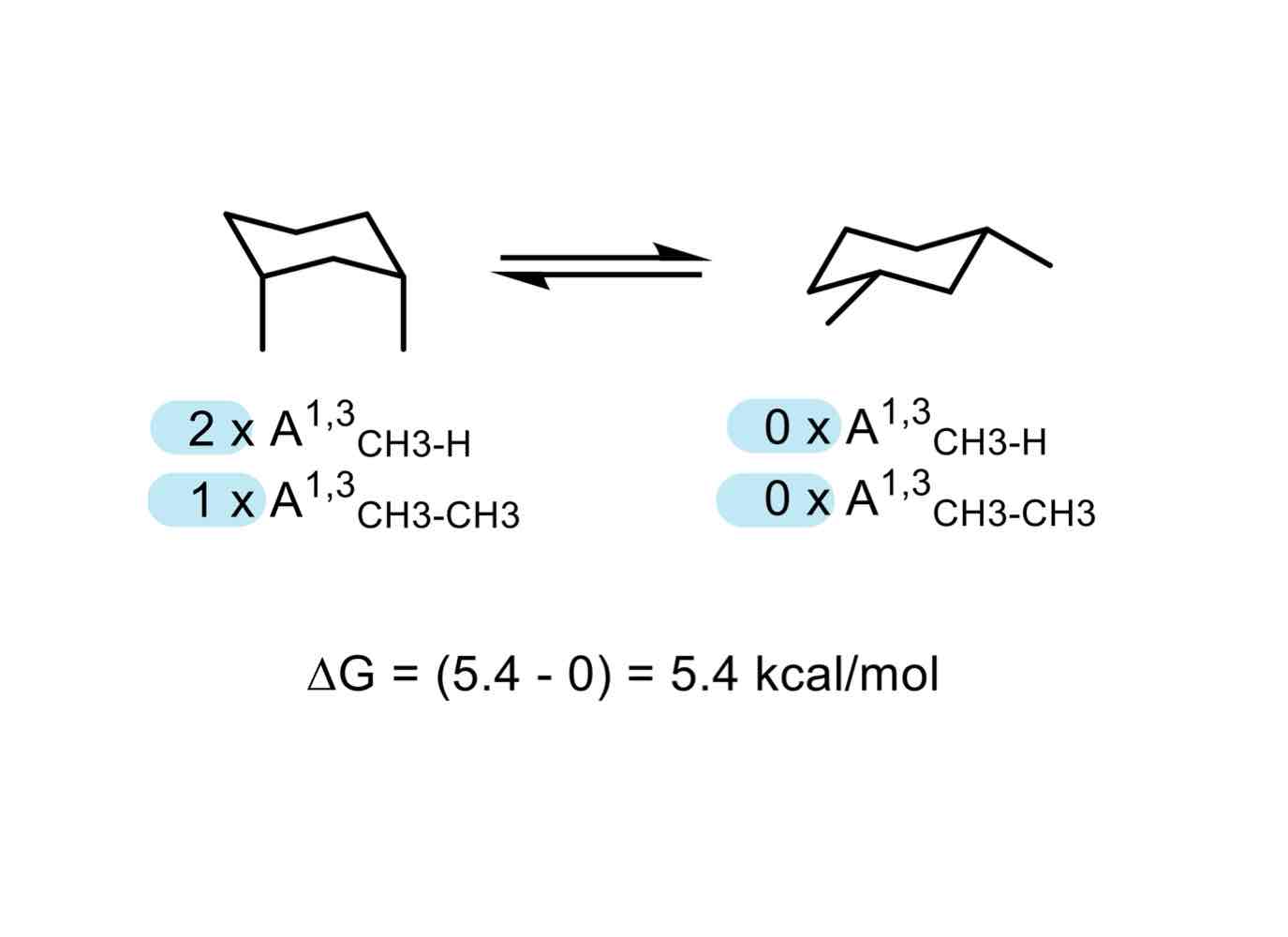
The _____ the substituent, the more preferred the ________ position.
The bigger the substituent, the more preferred the equatorial position.
We don’t need to know the exact values that give us the increase or decrease of energy to understand which conformation of a cyclic system works best. We just need to know… what?
The number of interactions there are.
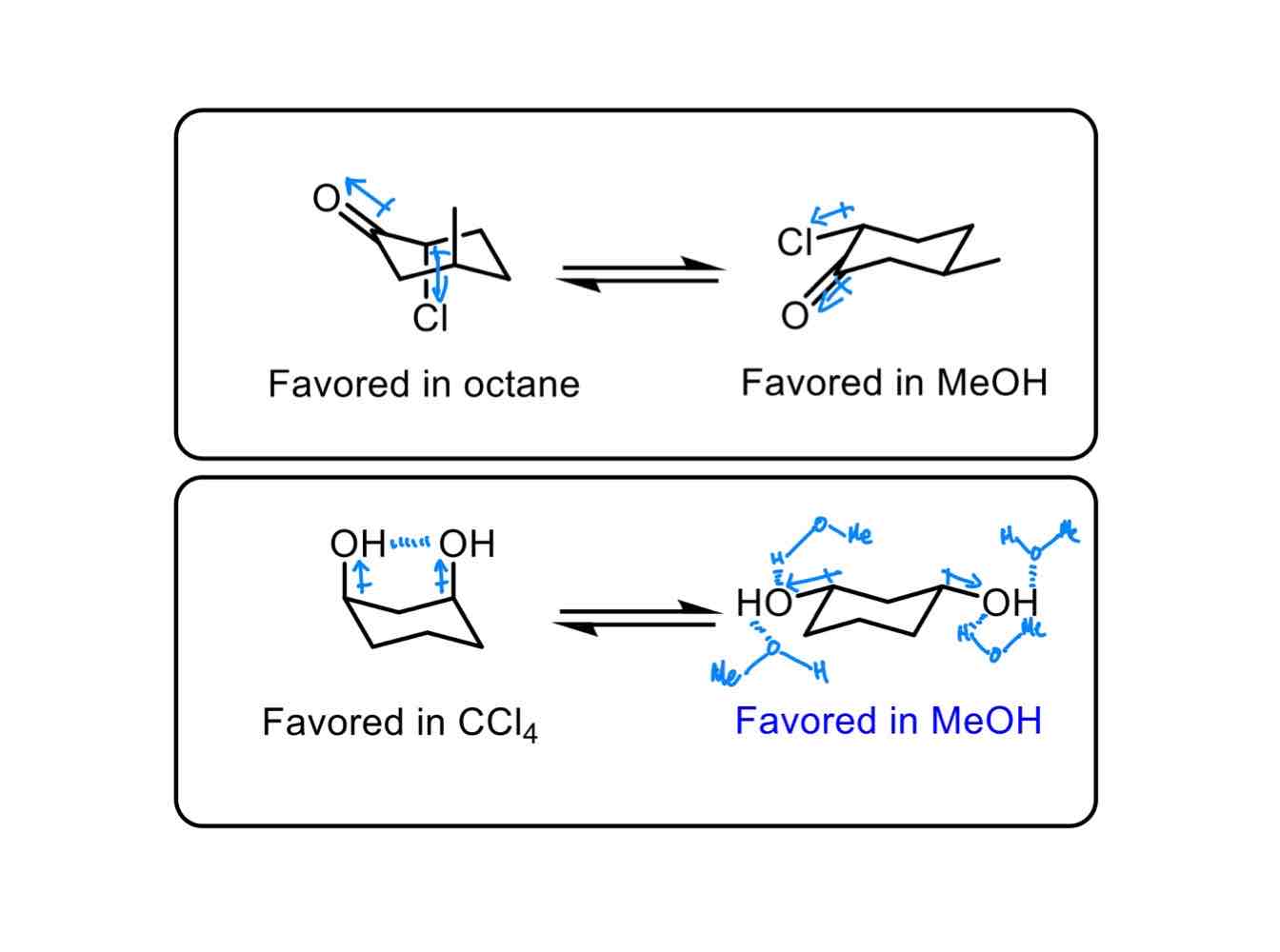
Other than the molecules at hand, just switching the _______ can turn an impossible, non-favoured reaction to a possible, favoured reaction.
Other than the molecules at hand, just switching the solvent can turn an impossible, non-favoured reaction to a possible, favoured reaction.
As discussed in class, give one of two ways of how changing the solvent can affect a reaction.
If you change a non-polar solvent to a polar solvent with a molecule that can adopt a conformation wherein it can turn from non-polar to polar, its polar “conformation” will assert complete dominance, as like dissolves like.
If you change a solvent that cannot hydrogen-bond to a solvent that can hydrogen-bond with a molecule that can adopt conformations wherein hydrogen bonds are minimized and maximized, its hydrogen bond-maximized “conformation” will assert dominance.
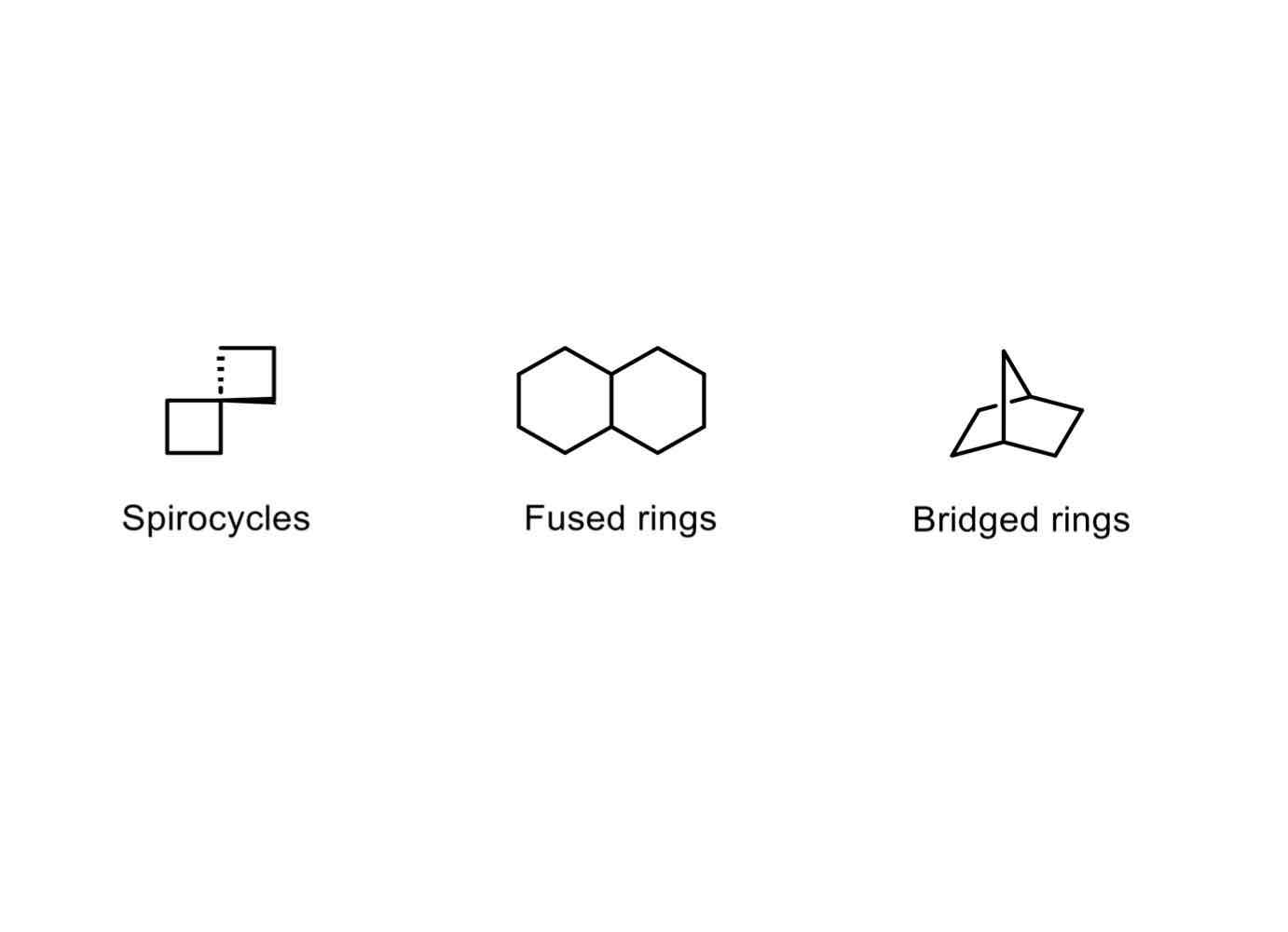
There are three types of bicyclic systems. What are they? Briefly differentiate them.
Spirocycles, which are fused by a single atom.
Fused rings, which are fused by multiple atoms.
Bridged rings, which are fused by a “bridge.”
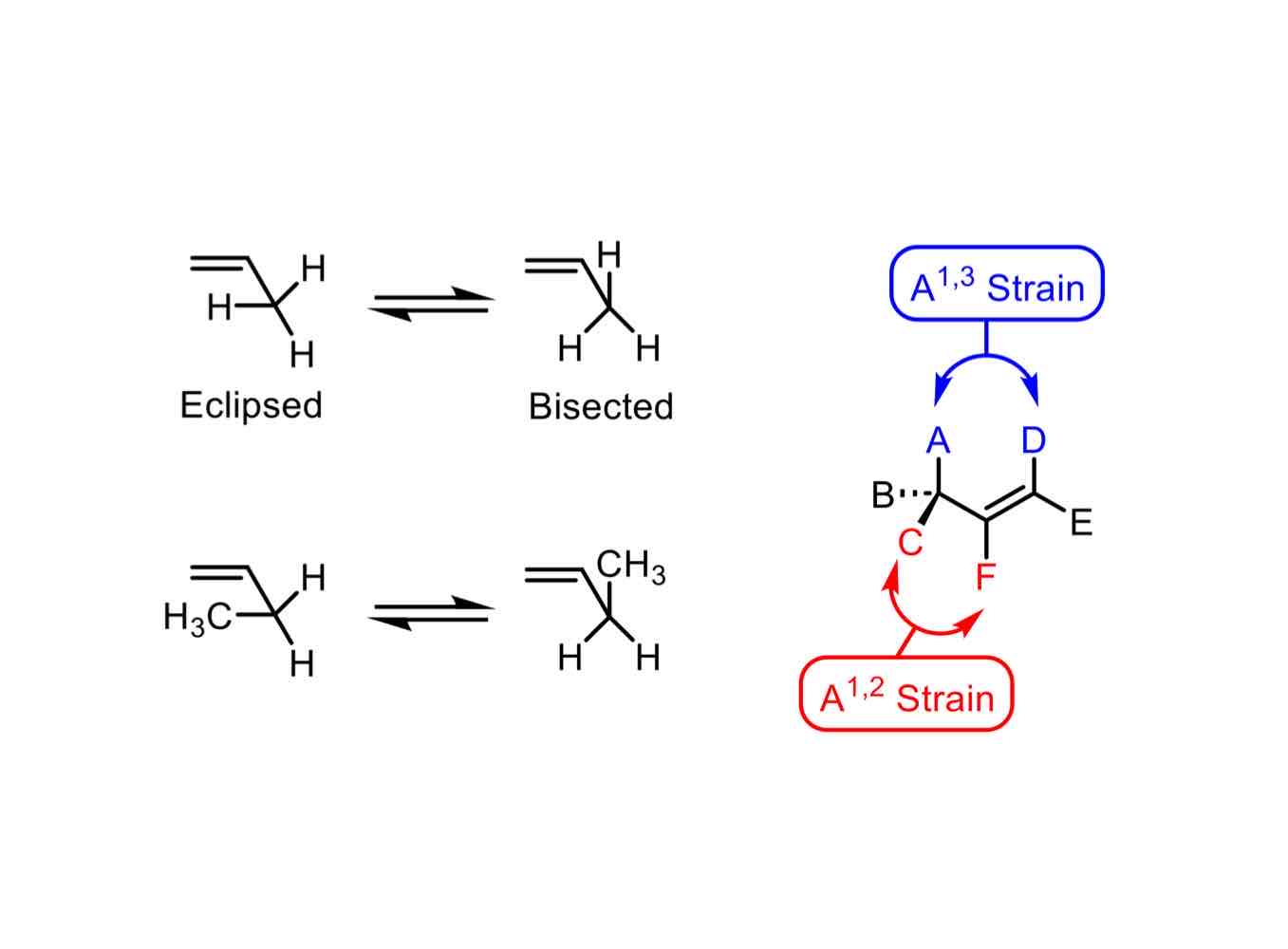
What type of strain is allylic strain? What causes allylic strain?
Steric strain caused by the steric interaction between substituents on either end of a locked alkene system.
What is Bredt’s rule?
In a bicyclic molecule, you cannot have a double bond connected to any bridgehead atom. If completely not being angle to have a double bond, then it requires a lot of energy.
Why can’t you have a double bond connected to any bridgehead atom, as per Bredt’s rule?
p-orbitals are not aligned with each other to overlap and make a π—bond. p-orbitals are 90º away from each other, and overlap isn’t possible.
What is are isomers?
Molecules with the same atoms but differ regardless.
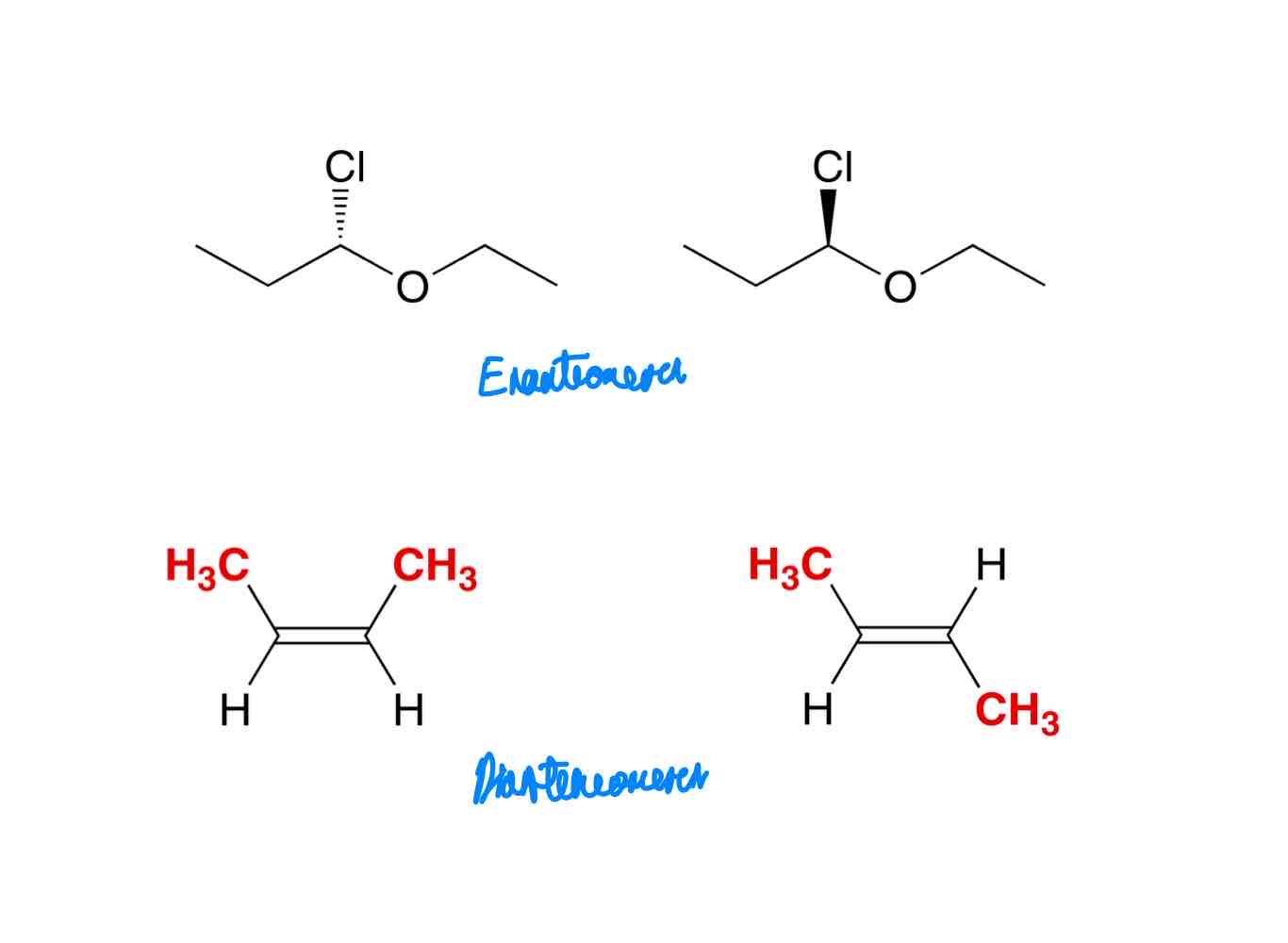
Differentiate the two kinds of isomers: constitutional isomers and stereoisomers.
Constitutional isomers: same molecular formula, different constitution.
Stereoisomers: same molecular formula, same constitution, different arrangement in space.
Differentiate the three types of stereoisomers: conformational isomers, enantiomers, and diastereomers.
Conformational isomers, a.k.a. conformers, are stereoisomers that interconvert by low energy processes like bond rotations and chair flips.
Enantiomers are stereoisomers that are non-superimposable mirror images.
Diastereomers are stereoisomers that are non-superimposable non-mirror images.
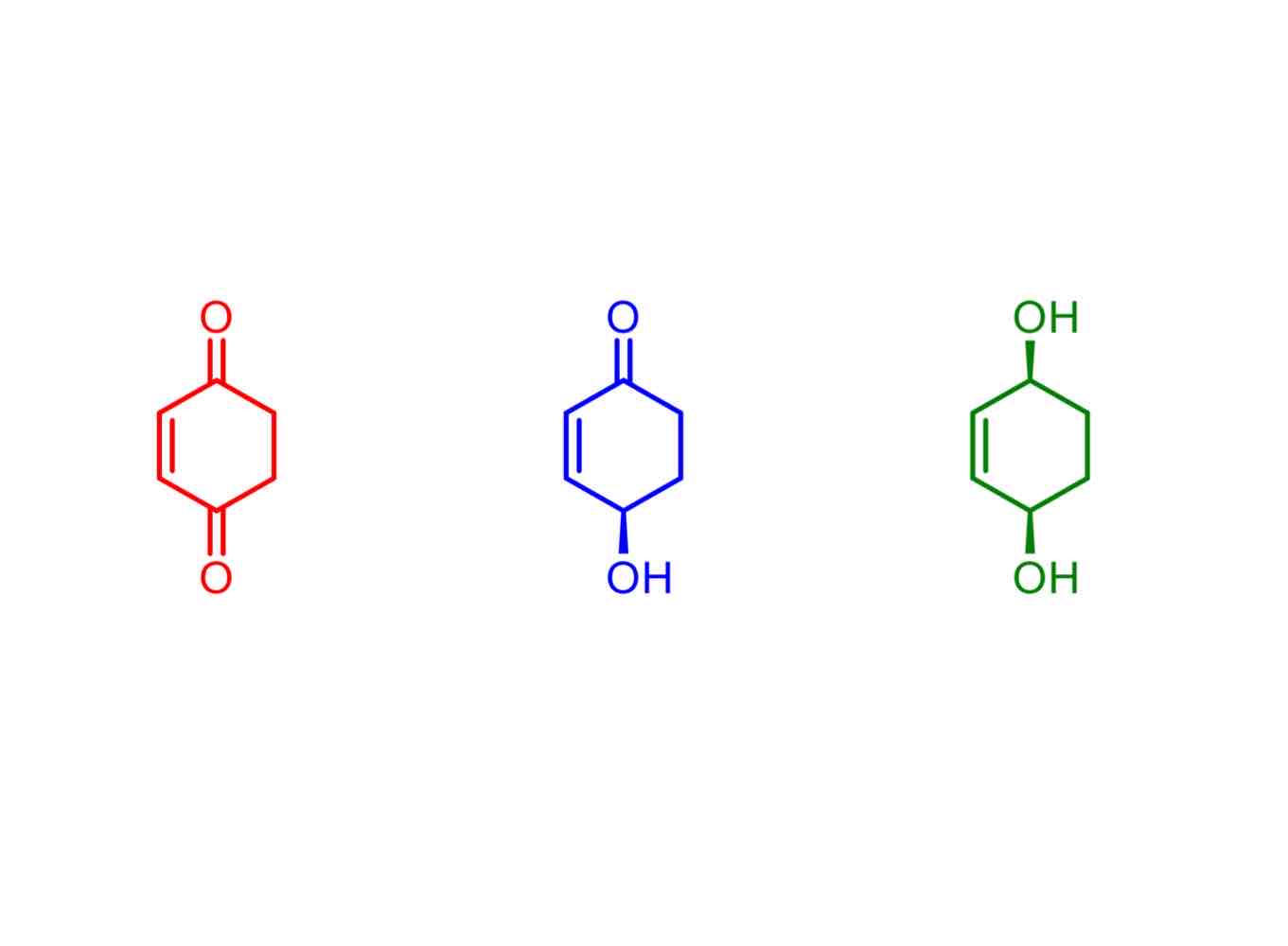
Differentiate these three: achrial, chrial, and meso molecules. Additionally, in what specific field of chemistry do we use these terms?
Achiral molecules: A molecule that is superimposable on its mirror image.
Chiral molecules: A molecule that is not superimposable on its mirror image.
Meso molecules: A molecule that is superimposable on its mirror image by virtue of an internal plane of symmetry.
These terms are talked about in the field of stereochemistry.
What is a prochiral species?
A proton or group that leads to a chiral centre once modified or substituted.