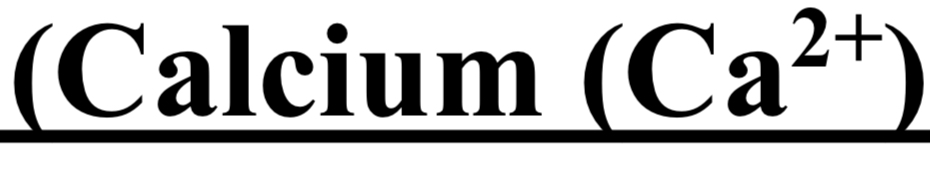analytical chem cations
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
TEST FOR GROUP VI (ammonium NH4+)
cation + dil. NaOH → NH3 odor
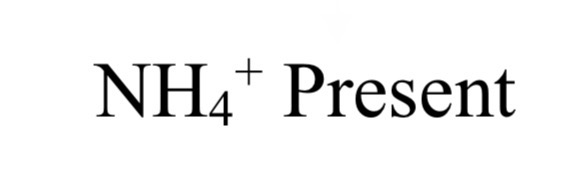
TEST FOR GROUP I (Silver Ag+ & Lead Pb2+)
cation + dil. HCI → white ppt → Ag or Pb is present
hot distilled water → ppt doesnt dissolve
NH4OH + dil. HNO3 → white ppt
NH4OH + KI → yellow ppt
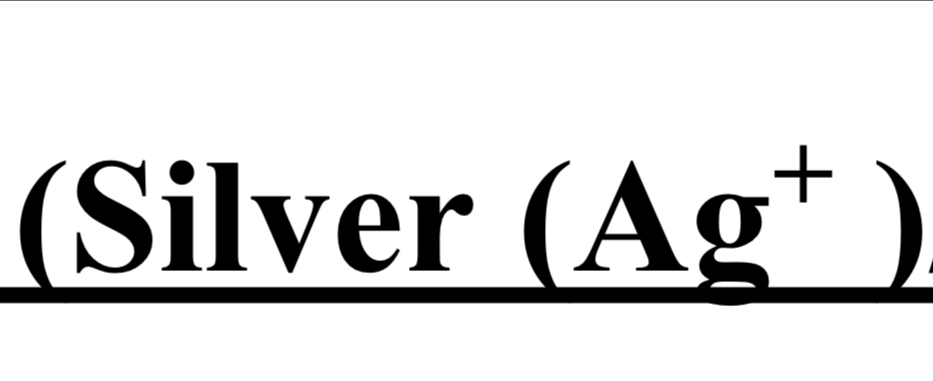
TEST FOR GROUP I (Silver Ag+ / Lead Pb2+)
cation + dil. HCI → white ppt → Ag or Pb is present
hot distilled water → ppt dissolves
dil CH3COOH + K2CrO4 → yellow ppt
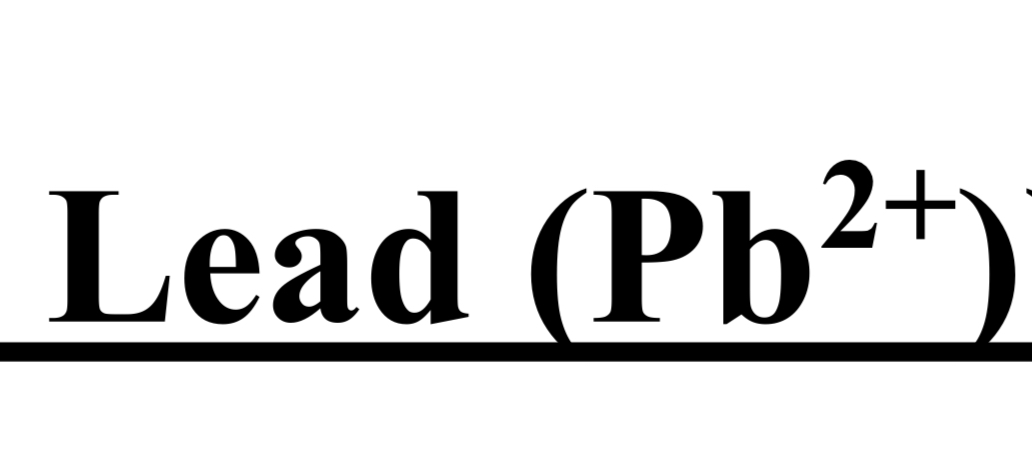
TEST FOR GROUP II (Cadmium 2+ / Copper 2+)
cation + dil. H2S → yellow ppt

TEST FOR GROUP II (Cadmium 2+ / Copper 2+)
cation + dil. H2S → black ppt
dil. HNO3 + heat → black crust + clear solution
conc NH4OH → blue solution
1- dil CH3COOH + potassium ferrocyanide → reddish brown ppt
2- dil HNO3 + KI → white ppt + yellowish brown solution
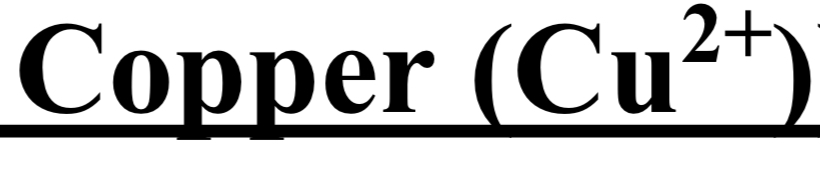
TEST FOR GROUP III ( Ferric Fe 3+ / Chromium Cr 3+ )
cation + NH4CI + dil NH4OH → greyish green ppt
dil NaOH + H2O2 + heat till no bubbles
dil CH3COOH + lead acetate → yellow ppt

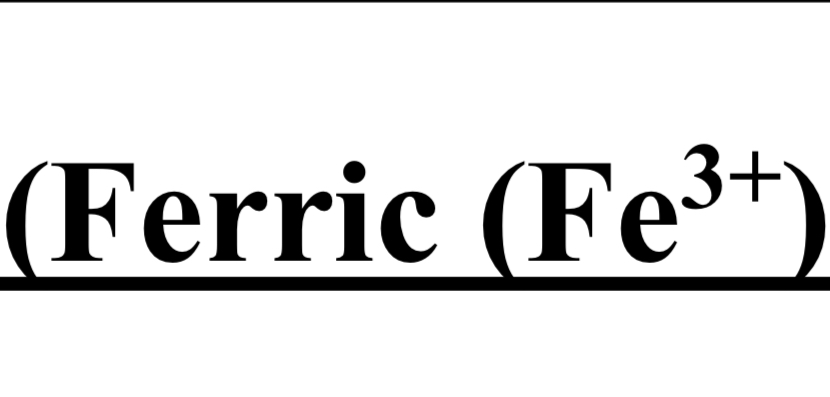
TEST FOR GROUP III ( Ferric Fe 3+ / Chromium Cr 3+ )
cation + NH4CI + dil NH4OH → brown ppt
dil HNO3 + heat + NH4SCN → blood red color
TEST FOR GROUP V ( Calcium Ca 2+ / Barium Ba 2+ )
cation + NH4CI + NH4OH + (NH4)2CO3 → white ppt
dil CH3COOH + heat → ppt dissolves
split solution
1- K2CrO4 → yellow ppt
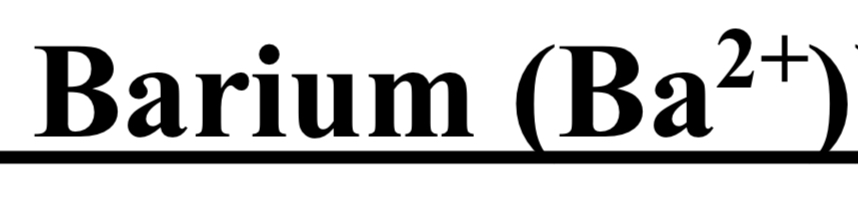
TEST FOR GROUP V ( Calcium Ca 2+ / Barium Ba 2+ )
cation + NH4CI + NH4OH + (NH4)2CO3 → white ppt
dil CH3COOH + heat → ppt dissolves
split solution
1- K2CrO4 → no observation
2- NH4OH + ammonium oxalate → white ppt