Organic Chemistry (AS + A-Level)
1/159
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
160 Terms
Alkanes
Saturated hydrocarbons
General formula = CnH2n+2
Fractional distillation of alkanes
Alkanes are found in crude oil (mixture of different lengths of hydrocarbons)
Column has temp gradient: cooler at top, hotter at bottom
Longer chain hydrocarbons condense
Uses of crude oil
Gas - stove
Kerosene - jet fuel
Bitumen - roofing
Diesel oil, petrol, fuel oil
Purpose of cracking
Heavier fractions can be cracked into higher demand, lighter fractions
Thermal cracking conditions + common products
Conditions: high temperature (1000o C) + high pressure (70 atm)
Products are usually alkenes (used to make polymers)
Catalytic cracking conditions + common products
Conditions: high temperature (40o C) + slight pressure used with zeolite catalyst
Zeolite lowers temp + pressure needed so lower costs and faster process
Products are usually aromatic hydrocarbons (useful for fuels in vehicles)
Complete combustion of alkanes
Burn in oxygen to form CO2 and H2O
Alkanes are good fuels as most burn readily to produce large amounts of energy
Used to power vehicles + most electricity
Incomplete combustion of alkanes
Burn in limited O2 supply to form CO (carbon monoxide) and soot
CO is poisonous: binds to haemoglobin in blood which prevents O2 binding, can be removed using catalytic converter
Soot causes breathing problems
Global warming
Burning fossil fuels produces CO2 (greenhouse gas)
CO2 absorbs IR from sun but emits some back to earth
Removal of sulfur dioxide from flue gases
Using CaO: CaO (s) + 2H2O (l) + SO2 (g) → CaSO3 (s) + 2H2O (l)
Using CaCO3: CaCO3 (s) + 2H2O (l) + SO2 (g) → CaSO3 (s) + 2H2O (l) + CO2 (g)
Photochemical smog
Oxides of nitrogen made from N and O2 in air combining under high pressure + temp e.g., car engines (catalytic converts can reduce NOx going into atmosphere)
Harms respiratory system + toxic
Acid rain
Burning fossil fuels releases SO2
Reacts w H2O to form H2SO4 which falls as acid rain: damages plants, kills fish, causes erosion
Wet scrubbing can remove SO2 by neutralising with CaCO3
Initiation
Radicals are produced normally using UV light
Bond breaks, producing 2 radicals
E.g., Cl-Cl → Cl•
Propogation
Radical reacts with non-radical, new radicals are created which react with more non-radicals
E.g., CH3CH3 + Cl• → •CH2CH3 + HCl
Termination
When 2 radicals react, they form non-radical molecule which ends chain reaction
E.g., •CH2CH3 + Cl• → ClCH2CH3
Halogenoalkanes
Alkanes with one or more halogens attached to it
BP trends of halogenoalkanes
Increases down the group
More electrons = stronger vdw forces so more energy needed to overcome
Bond polarity of halogenoalkanes
Have a polar bond + attacked by nucleophiles
Halogens are more electronegative than carbon so pull electrons towards themselves
Nucleophile
Substance that is an electron pair donor e.g., NH3, OH-, CN-
Halogenoalkane reaction with hydroxide ions (ns)
Via nucleophilic substitution in warm aqueous NaOH + under reflux
1) Nucleophile attacks δ+ carbon
2) Nucleophile replaced halogen
R-X + NaOH → ROH + NaX
Halogenoalkane reaction with cyanide ions
Via nucleophilic substitution in warm ethanolic KCN + under reflux, creeates nitriles
1) Nucleophile attacks δ+ carbon
2) Nucleophile replaces halogen
R-X + KCN → RCN + KX
Halogenoalkane reaction with ammonia
Via nucleophilic substitution in warm ethanolic NH3 (excess)
1) Ammonia attacks δ+ carbon
2) Ammonia replaces halogen, forming intermediate
3) Another ammonia acts as base by reacting w hydrogen
4) Amine produced and ammonium ion produced
Halogenoalkane reactivity
Increases down the group
Bond strength/bond enthalpy determines reactivity, not bond polarity
Halogenoalkane reaction with hydroxide ions (e)
Via elimination in warm ethanolic NaOH under reflux
1) OH- attacks hydrogen on carbon adjacted to carbon with halogen
2) OH- acts as a base, forming water
3) Electrons in bond move to form double bond between two carbons
4) C-X breaks, both electrons move from bond to halogen
Chlorofluorocarbons (CFCs)
Molecules that have had all their hydrogens replaced by chlorine and fluorine
Effect of CFCs on ozone (O3)
Breaks down O3 in atmosphere
C-Cl bond broken by UV radiation - radicals formed, catalyse breakdown of ozone
C-Cl bonds are broken easiest by UV as they have lowest bond enthalpy
Equations for CFCs destroying ozone
1) Initiation
CCl3F → CCl2F + Cl•
2) Propogation
Cl• + O3 → O2 + ClO•
ClO• + O3 → 2O2 + Cl•
3) Termination
Cl• + Cl• → Cl2
Restricting use of CFCs
CFCs are stable, unreactive, non-toxic + were refrigerants but destroy ozone
HFCs used instead as they do not have chlorine
Alkenes
Unsaturated hydrocarbons, general formula = CnH2n
Cycloalkenes
Have 2 fewer hydrogens than alkenes, general formula = CnH2n-2
Alkenes electrophilic addition reactions
Alkenes are attacked by electrophiles due to double bond
Double bond has high electron density
Electrophile
Electron pair acceptor e.g., NO2+, H+, H-Br, H2SO4
Bromine test for alkenes
Causes color change from brown-orange to colourless
Br is electrophile + adds to alkene to form dibromoalkene
Alkenes - addition of hydrogen halides
React with hydrogen halides to form halogenoalkanes
Reacting with unsymmetrical alkenes forms 2 different products (amount of products depends on stability of carbocation intermediate)
More alkyl groups bonded to carbocation = more stable intermediate
Alkyl groups push electrons towards carbocation + stabilises it
Stability: Tertiary > Secondary > Primary
Alkenes - addition of sulfuric acid
React with cold, conc H2SO4 to form alkyl hydrogen sulfates
If water added, alcohol will reform
E.g., H2C=CH2 + H2SO4 → CH3CH2OSO2OH
CH3CH2OH + H2O → CH3CH2OH + H2SO4
Alkenes into addition polymers
Alkenes are monomers with join to form addition polymers - can be natural (proteins/rubber) or synthetic (poly(ethene))
Polyalkene properties
Most polyalkene chains are non-polar so only vdw forces
Longer chain + closer together = more vdw
Some polyalkenes have halogens (can have permanent dipole-dipole)
Plasticisers
Are added to polymers to increase flexibility - slide between polymer chains, pushing them apart, weakening intermolecular forces so chains can slide over each other + bend
PVC and plasticisers
PVC made from long, closely-packed polymer chains that are hard but brittle (used in drain pipes)
PVC w plasticisers is more flexible (used for electrical insulation + clothing)
Alcohols
Have functional group -OH (hydroxyl), general formula = CnH2n+1OH
Dehydration of alcohols/hydration of alkenes
e.g., ethanol → ethene + water (use of acid catalyst)
Alkenes can be made from ethanols sustainably if alcohol has been made via fermentation of glucose from plants
Elimination reactions of alcohols
Dehydration of non-primary alcohols can lead to 2 different alkenes - C=C can be formed on either side of carbon with OH
1) Lone pair on O will attach to H+ from acid catalyst
2) Intermediate formed has a +ve charge on O - O pulls electrons in C-O bond strongly to break bond, leaving unstable carbocation intermediate
3) Carbocation loses H+ - electrons in C-H move to form C=C bond
Making alcohols from hydration of alkenes
Steam and acid catalyst used
E.g., ethanol:
1) React steam + ethene with phosphoric acid catalyst
2) Temp of 300oC and 60 atm
Making alcohols from fermentation
Making ethanol via fermentation uses renewable source of glucose from plants
Uses yeast in anaerobic conditions - reaction is exothermic
Requires little equipment + uses renewable resources so is cheap
Biofuels
Made from dead biological matter
Ethanol is being used as fuel in countries with good supply of sugar cane
Sugar is fermented to produce alcohol
Advantages of biofuels
Renewable so more sustainable than crude oil
Produce CO2 when burnt but classed as carbon neutral because carbon dioxide absorbed by sugar cane when growing
Disadvantages of biofuels
Expensive to convert existing petrol engines to take fuels w higher conc of ethanol
Land could be used to grow food rather than make fuel, could cause food shortages in countries growing sugar canes
Process of fermentation to make alcohol
1) Plants taken in CO2 + make glucose via photosynthesis
6CO2 + 6H2O → C6H12O6 + 6O2
2) During fermentation, ethanol is produced
C6H12O6 → 2CO2 + 2C2H5OH
3) Combustion of ethanol
2C2H5OH + 6O2 → 4CO2 + 6H2O
Oxidation of alcohols
Used acidified potassium dichromate, K2Cr2O7
Primary alcohols oxidise to aldehydes then carboxylic acids
Secondary alcohols oxidise to ketones
Tertiary alcohols cannot be oxidised
Mass spectrometry
Used to find relative molecular mass of compound
Peaks show fragments of original molecule
Last peak = M+1 peak (same as relative molecular mass of molecule)
m/z is mass divided by charge
Infrared spectroscopy
Uses infrared radiation to increase vibrational energy of covalent bonds in sample
Frequency depends on atoms either side of bond + position of bond in molecule
Purpose of fingerprint region in IR spectroscopy
Can compare fingerprint region against known library of spectra to identiy molecule
Extra peaks in fingerprint region indicates impurities in sample
Infrared and global warming
1) EM radiation from sun reaches earth + is absorbed by land and sea - some is re-emitted as infrared
2) Greenhouse gases absorb radiation + re-emit this back towards earth - covalent bonds absorb radiation
Optical isomerism
Form of stereoisomerism - have the same structural formula but different arrangement of atoms in space
Optical isomers
Are mirror images of each other + have chiral carbon atom
Chiral molecule
4 different groups attached to C atom; can be arranged in two different ways (enantiomers)
Enantiomers
Mirror images to each other + non-superimposable (do not overlap)
Identifying optically active isomers
Use plane-polarised light
One enantiomer rotates light clockwise, other rotates light anticlockwise
Racemic mixture/racemates
Equal amounts of each enantiomer - does not rotate plane polarised light
Molecules with planar profiles (e.g., double bonds in C=C or C=O) can make racemic products
Planar nature so even chance of nucleophile attacking from top or bottom so 50/50 mixture
Aldehydes
Have carbonyl group (C=O) on an end carbon, ends with -al
E.g., propanal CH3CH2CHO
Ketones
Have carbonyl group (C=O) on inner carbon, ends with -one
E.g., propanone CH3COCH3
Oxidation of aldehydes and ketones
Aldehydes are readily oxidised into carboxylic acids
Ketones do not oxidise
Testing for aldehydes + ketones - Tollen’s
1) Add few drops of NaOH to silver nitrate solution (pale brown ppt formed)
2) Add few drops of dilute ammonia until ppt dissolves
Aldehydes: silver mirror formed
Ketones: no silver ppt
Testing for aldehydes + ketones - Fehling’s
Aldehydes: goes from blue solution to brick red ppt
Ketones: remains blue
Reduction of aldehydes and ketones
Use NaBH4 in aqueous solution
Aldehydes: reduce to primary alcohol
Ketones: reduce to secondary alcohol
Nucleophilic addition with aldehydes and ketones
1) Nu attacks carbonyl carbon to form a -ve charged intermediate which quickly reacts with proton
Aldehydes + ketones with KCN
Produces hydroxynitriles via nucleophilic addition
1) CN- ion attacks C=O and adds on to make hydroxynitrile (with OH and CN group)
Aldehyde: RCHO + KCN + H+ → RCH(OH)CN + K+
Ketone: RCOR’ + KCN + H+ → RCR(OH)CN + K+
Using KCN
Risks: irritant, forms toxic gas (HCN)
Ways to reduce risk: wear lab coat, wear safety goggles, use fume cupboard, wear gloves
Carboxylic acids
Have carboxyl (-COOH) functional group; contains carbonyl (C=O) and hydroxyl (O-H) group, general formula = CnH2n+1COOH (COOH always on end of molecule)
Reactions of carboxylic acids
Weak acids - react with carbonates to form CO2
Partially dissociate to form H+ ion and carboxylate ion
React with carbonates to form salt, carbon dioxide gas and water
E.g., 2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
Esters
General formula RCOOR’, formed from:
Carboxylic acid + alcohol (acid catalyst) → ester + water
Acid anhydride + alcohol → ester + carboxylic acid
Naming of esters
First half = alcohol, second part = acid
E.g., methanol + ethanoic acid → methyl ethanoate
Uses of esters
1) Perfumes + food flavorings - some have sweet smells
2) Solvents → are polar so other polar compounds can dissolve, have low bps + evaporate easily so valuable for glue
3) Plasticisers
Can make plastics more flexible during polymerisation, penetrates polymer chains + increases distance between them
Acid hydrolysis of ester
Ester → Carboxylic Acid + Alcohol
Under reflux, heat, dilute acid (HCl, H2SO4)
Equilibrium established/does not go to completion
Base hydrolysis of ester
Ester → Carboxylate salt + alcohol
Under reflux, heat, dilute alkali (NaOH)
Reaction is irreversible/goes to completion
Fats and oils
Glycerol (alcohol) + fatty acids (carboxylic acids) → ester (makes fats and oils)
Glycerol (propane-1,2,3-triol) reacts w long chain fatty acids which can be saturated and unsaturated
Vegetable oils v animal fats
Vegetable oils: unsaturated hydrocarbon chains, not straight so cannot pack closely together so low vdw forces
Animal fats: saturated, straight so can pack closely so high vdw forces
Soap from animal fats/vegetable oils
Animals fats/vegetable oils can be hydrolysed by heating with NaOH to form soap
Fat + NaOH → glycerol + crude soap (saponification)
Biodiesel from vegetable oils
Biodiesel → mixture of fatty acids made from methyl esters + can be made by rapeseed oil
Vegetable oils can be converted to biodiesel by reaction with methanol + using KOH as catalyst
Triglyceride + methanol → fatty acid methyl esters + glycerol
Acyl chlorides
Have functional group (-CO-Cl) which contains acyl group (COCl), always at end of molecule
Reaction of acyl chloride with water
Acyl chloride + water → carboxylic acid
E.g., ethanoyl chloride with water
CH3COCl + H2O → CH3COOH + HCl
Vigorous reaction, white misty fumes of HCl produced
Reaction of acyl chloride with ammonia
Acyl chloride + ammonia → amide
E.g., ethanoyl chloride with ammonia
CH3COCl + NH3 → CH3CONH2 + HCl
Vigorous reaction, white misty fumes of HCl gas
Reaction of acyl chloride with alcohol
Acyl chloride + alcohol → ester
E.g., ethanoyl chloride with methanol
CH3COCl + CH3OH → CH3COOCH3 + HCl
Vigorous reaction, white misty fumes of HCl
Reaction of acyl chloride with primary amine
Acyl chloride + primary amine → n-substituted amide
E.g., ethanoyl chloride with primary amine
CH3COCl + CH3NH2 → CH3CONHCH3 + HCl
Vigorous reaction, white misty fumes of HCl
Acid anhydrides
Molecules made from 2 carboxylic acids that are the same
e.g., ethanoic acid + ethanoic acid → ethanoic anhydride
Reaction of acid anhydride with water
Acid anhydride + water → carboxylic acids
E.g., ethanoic anhydride with water
C4H6O3 + H2O → CH3COOH
Reaction of acid anhydride with ammonia
Acid anhydride + ammonia → amide
E.g., ethanoic anhydride with ammonia
C4H6O3 + NH3 → CH3CONH2 + CH3COOH
Reaction of acid anhydride with alcohol
Acid anhydride + alcohol → ester
E.g., ethanoic anhydride with methanol
C4H6O3 + CH3OH → CH3COOH3 + CH3OOH
Reaction of acid anhydride with primary amine
Acid anhydride + primary amine → n-substituted amides
E.g., ethanoic anhydride with primary amine
C4H6O3 + CH3NH2 → CH3CONHCH3 + CH3COOH
Acyl chlorides and nucleophilic addition-elimination
Acyl chlorides have strong δ+ on carbon which can be attacked by nucleophiles (due to Cl being electronegative)
1) Addition of nucleophile across C=O bond
2) Elimination of small molecule such as HCl or H2O
Nucleophiles such as NH3, H2O, CH3NH2
Making aspirin
Salicylic acid + ethanoic anhydride → aspirin + ethanoic acid
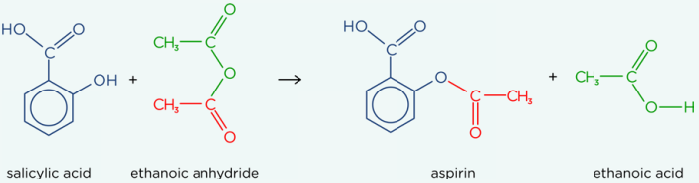
Why use ethanoic anhydride than ethanoyl chloride for aspirin production
Cheaper, less corrosive, safer, produces less toxic by product, does not react with water readily
Benzene
Cyclic, planar molecule with formula C6H6
Structure of benzene
Each C is bonded to 2 other Cs + 1 H
Final lone electron is in p-orbital which sticks out above + below planar ring
Lone electrons in p-orbitals combine to form delocalised ring of electrons
All C-C bonds in molecule are same due to delocalised electron structure
Stability of benzene
Benzene is more stable than theoretical alternative cyclohexa-1,3,5-triene
Benzene (-360 kJ mol) has lower enthalpy change than cyclohexa-1,3,5-triene (-120 kJ mol) so more energy required to break bonds + more energy released to form bonds
Arene
Aromatic compound that contains benzene ring
Named in 2 ways:
1) Benzene at end e.g., bromobenzene
2) Phenyl, C6H5, as functional group e.g., phenylamine
Reactions of arenes
Benzene has high electron density due to delocalised ring of electrons (attractive to electrophiles)
Benzene is stable so does not undergo electrophilic addition, it undergoes electrophilic substitution so ring of electron is not disturbed
Friedel-Crafts acylation
Acyl group (RCO-) is added onto benzene so structure becomes weaker so easier to modify + make into useful products
Electrophile must have very strong +ve charge to add onto benzene ring
AlCl3 (halogen carrier) is used as a catalyst to produce stronger electrophile from acyl group
Nitration of benzene
Nitrobenzene made from heating benzene w conc HNO3 and H2SO4 but powerful electrophile needed first
Used in dyes + pharmaceuticals
Used in TNT
1) HNO3 + H2SO4 → H2NO3+ + HSO4- + NO2+ + HSO4-
2) Electron ring attracted to NO2+ then replaces H with NO2
3) HSO4- + H+ → H2SO4
Amine
Derived from ammonia molecules + contain a nitrogen atom where hydrogens are replaced with an organic group
Primary amine = 1 organic group
Secondary amine = 2 organic groups
Tertiary amine = 3 organic groups
Quarternary amine = 4 organic groups (exists as +ve salt)