Dynamic equilibrium and le Chatelier's principle
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Define a closed system
A system that is isolated from its surroundings, so the temperature, pressure and concentrations of reactants and products are unaffected by outside influences
Define dynamic equilibrium
Equilibrium that exists:
In a closed system
When the rate of the forward reaction is equal to the rate of the reverse reaction
And the concentrations of reactants and products do not change
What does le Chatelier’s principle state?
When a system in dynamic equilibrium is subject to a change, the position of equilibrium will shift to minimise that change
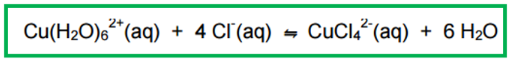
What are the colours of the compounds in this experiment?
Cu(H2O)62+(aq) is blue
CuCl42-(aq) is yellow
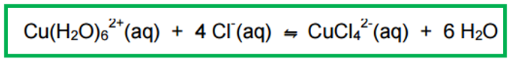
Explain the effect of:
Adding HCl
Adding H2O
Adding HCl increases the concentration of Cl-, so the position of equilibrium shifts to decrease the concentration of Cl-, moving to the right
The reaction turns yellow as CuCl42- is formed
Adding H2O increases its concentration so the position of equilibrium shifts to the left, favouring the backwards reaction to turn the mixture blue

Explain the effect of:
Adding water
Adding concentrated HCl
Increased concentration of water, position of equilibrium shifts to decrease the concentration of water, equilibrium shifts left, pink
Increased concentration of Cl-, position of equilibrium shifts to decrease the concentration of Cl-, shifts right, blue

For this reaction, explain the results shown when:
Solution turns blue when put in water at 90C
Solution turns pink when put in ice
Increased temperature, position of equilibrium shifts to decrease temperature, reaction is endothermic forwards
Decreased temperature, position of equilibrium shifts to increase temperature, favouring exothermic reactiton, exothermic backwards
To which kinds of reactions will a change in pressure affect the position of equilibrium?
Reactions where gases are present
Give the equation for the Haber Process, and state whether this reaction is exothermic or endothermic
N2(g) + 3H2(g) ⇌ 2NH3(g) (exothermic forward reaction)
Using the Haber Process as an example, explain the effect of:
Increasing the pressure
Decreasing the pressure
N2(g) + 3H2(g) ⇌ 2NH3(g)
Increase pressure, position of equilibrium shifts to decrease pressure, favouring the side with less gas moles, position of equilibrium shifts to the right
Decreased pressure, position of equilibrium shifts to increase pressure, favours side with more gas moles, shifts to left
What effect does a catalyst have on the position of equilibrium?
A catalyst increases the rate of both the forward and reverse reaction in an equilibrium by the same amount, resulting in an unchanged position of equilibrium
What is the main aim of many important industrial chemical processes?
To make the highest possible yield of product at the least cost
What is the temperature used for the Haber Process and why is this a compromise temperature?
450C is used
In theory the forward reaction is favoured by a high pressure and low temperature
But a low temperature would decrease the rate, leading to a compromise temperature of 450C as this leads to a sufficiently fast rate without sending the position of equilibrium too far to the left
What pressure is used for the Haber Process and why is this a compromise?
200 atm is used
In theory a high pressure would favour the forward reaction
But a high pressure requires high energy to compress the gases, so a compromise 200atm is used due to the cost and safety implication
What catalyst is used for the Haber Process and why?
Finely divided iron for large surface area, and speeding up of the reaction rate
This is a heterogeneous catalyst
Only a small percentage of nitrogen and hydrogen is converted into ammonia. What is done to get around this problem?
Unreacted hydrogen and nitrogen are recycled repeatedly, so nearly all of it is eventually converted into ammonia
When steam is passed over heated carbon the following quilibrium is established:
C(s) + H2O ⇌ CO(g) + H2(g)
What would happen if the temperature dropped below 100 degrees C?
The gaseous H2O would condense to water
Equilibrium shifts to the left-hand side
CO and H2 converts to carbon and water