Cell Metabolism
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
cell metabolism
organism selection: need the right one for the job
cell has to efficiently make the product
genetic engineering allows engineers to add/remove genes to alter metabolic functions
differences in microbial metabolism due to:
genetic differences
differences in response to environment
example: S. cerevisiae
anaerobic = ethanol
aerobic = baker’s yeast
two key concepts:
catabolism: degrading a compound to smaller and simpler products
glucose to CO2 and H2O
anabolism: synthesis of more complex compounds
glucose to oxygen
ATP
how energy in biological systems is stored and transferred
contains high energy phosphate bonds
analog compounds of ATP also store and transfer high energy phosphate bonds
GTP
UTP
CTP
cellular energetics
ATP is needed in cells to drive energetically unfavorable reactions
eukaryotic cells use specialized membranes inside of energy-converting organelles (mitochondria) to produce most of their ATP
prokaryotic cells produce ATP in cytosol via glycolysis and in the cell wall
ATP:ADP ratios
ATP:ADP ratio within the cell must stay high
the ATP pool is used to drive energetically unfavorable reactions
if ATP levels begin to fall, energetically unfavorable reactions cease, the cell die
cyanide - blocks electron transport in the inner mitochondrial membrane
the ATP:ADP ratio must remain high
deltaG = deltaG0 + RT*ln{[ADP][Pi]/{ATP]}
at low [ATP], deltaG approaches zero
at low [ATP] many biosynthetic reactions wound begin to run backwards
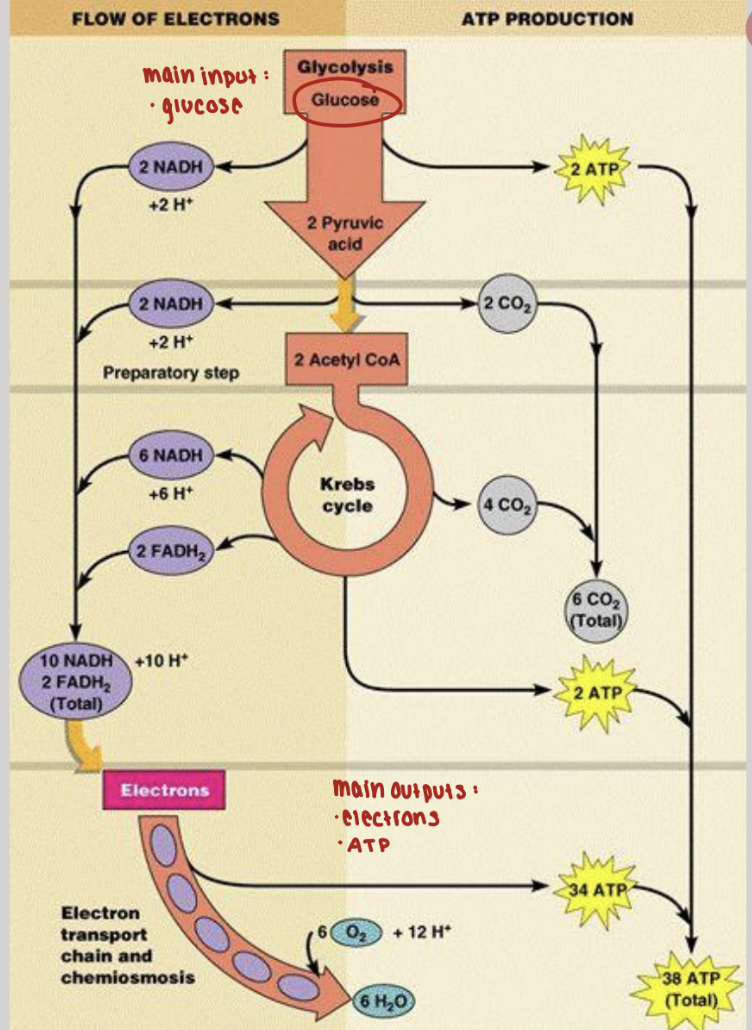
glucose metabolism
glucose is the major energy source for many organisms
aerobic catabolism of glucose has three phases
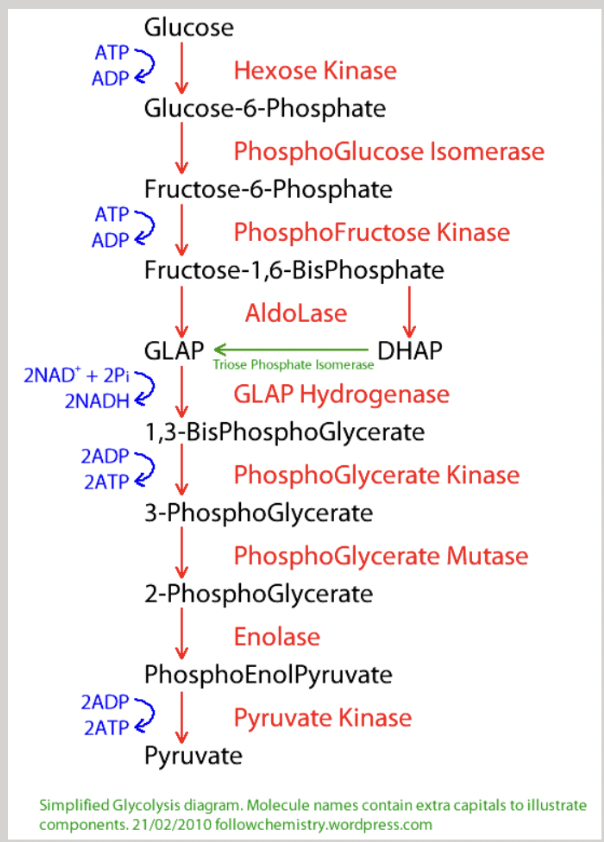
embden-meyerhof-parnas (EMP) pathway transforms glucose to pyruvate
citric acid cycle for conversion of pyruvate to CO2 and NADH
ETC for formation of ATP
mitochondria
occupy a large fraction of the cytoplasmic volume
essential for the evolution of higher eukaryotes
metabolism of sugars by mitochondria produces 15 times more ATP than glycolysis
enclosed by two specialized membrranes
outer membrane contains porin molecules to transport small molecules
inner membrane contains:
phospholipid cardiolipin: makes membrane especially impermeable to ions
transport proteins that transport molecule needed for enzymes within the matrix
matrix includes enzymes that metabolize pyruvate and fatty acids to produce acetyl CoA and those that oxidize acetyl CoA in the citric acid cycle
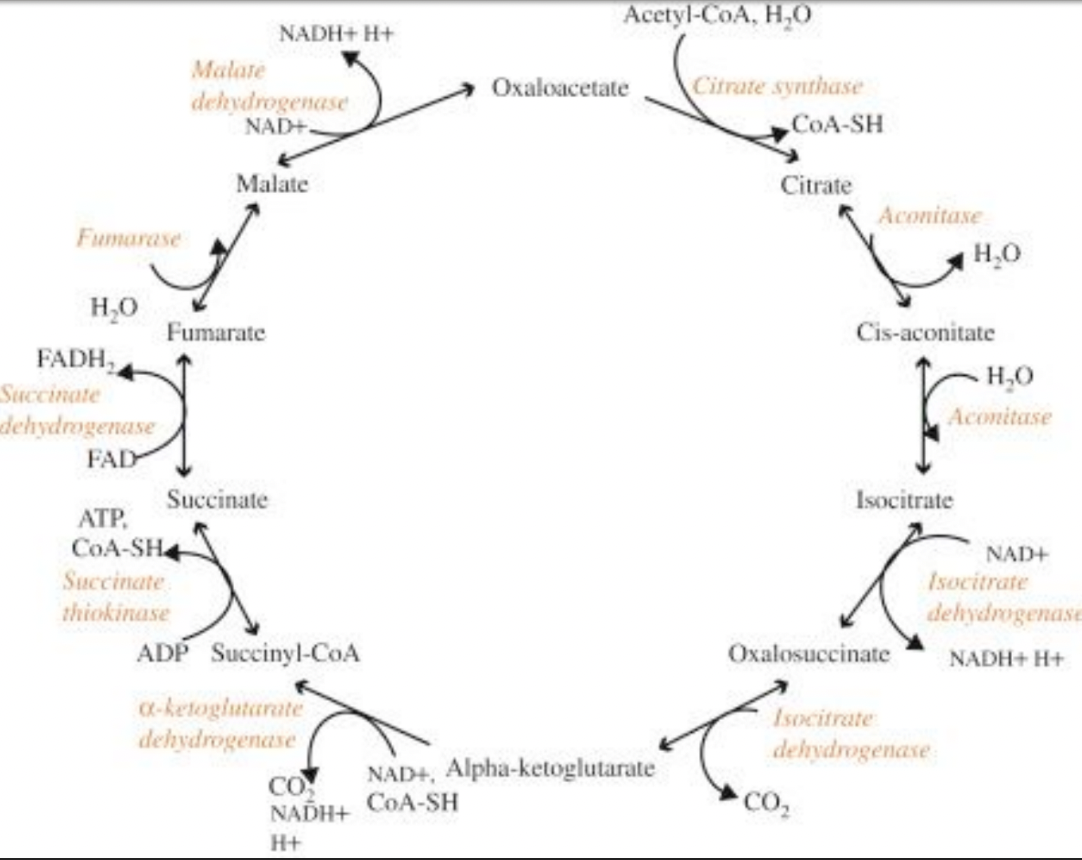
citric acid cycle
mitochondria use both pyruvate (glucose and other sugars) and fatty acids (fats) for fuel
pyruvate and fatty acids are transported across the inner mitochondrial membrane and converted to acetyl CoA by matrix enzymes
acetyl CoA is converted (via the citric acid cycle) to CO2 and high energy electrons
high energy electrons are carried by the activated carrier molecules NADH and FADH2
electrons are transferred to the inner mitochondrial membrane
NAD+ and FAD are regenerated
the CAC is considered part of aerobic metabolism, but does not use O2
the major roles of the TCA cycle are
to provide electrons for electron transport chain
supply C skeletons for AA synthesis
generate energy
respiration
in the final catabolic reactions on the inner mitochondrial membrane, O2 is used
electrons transferred by NADH and FADH2 to O2 through a series of electron carriers
ATP is formed
chemiosmotic coupling
common pathway used by cells to harness energy
chemical bond forming reactions that form ATP (chemi)
membrane transport process (osmotic)
occurs in two stages:
stage 1: high energy electrons are transferred between electron carriers embedded in the membrane; electron transfers release energy that is used to pump protons across the membrane and generate an electrochemical proton gradient
stage 2: H+ flows down its electrochemical gradient through ATP synthase (acts like a turbine), which catalyzes the synthesis of ATP from ADP and inorganic phosphate
electron transport chain
composed of both the membrane proteins and small molecules involved in electron transfer
in biological systems, electrons are carried from one site to another by diffusible molecules
mitochondria use NAD+, which picks up two electrons and an H+ molecule to form NADH
biological oxidation
respiratory chain harvests energy from the energetically favorable reaction: H2 +1/2O2 → H2O
reaction happens in many small steps so most of the released energy can be stored instead of lost as heat
hydrogen atoms are first separated into protons and electrons
electrons pass through a series of electron carriers in the inner mitochondrial membrane
at the end of the ETC protons are returned permanently when they neutralize the oxygen molecule
electron transport begins when the hydride ion is removed from NADH
converted to a proton and two electrons
H- → H+ + 2e-
the two electrons are passed to the first of many different electron carriers in the respiratory chain
primarily passed from one metal ion to another
metal ions are bound to transmembrane proteins that alter the affinity of the metal ions for electrons
each metal/protein complex in the chain has a greater electron affinity than the last
final transfer is to oxygen, which has the greatest electron affinity
electrons start with very high energy and gradually lose energy as the pass along the chain
energy storage
the energetically favorable transfer of electrons is coupled to:
oriented H+ uptake and release
allosteric changes in energy-converting protein pumps
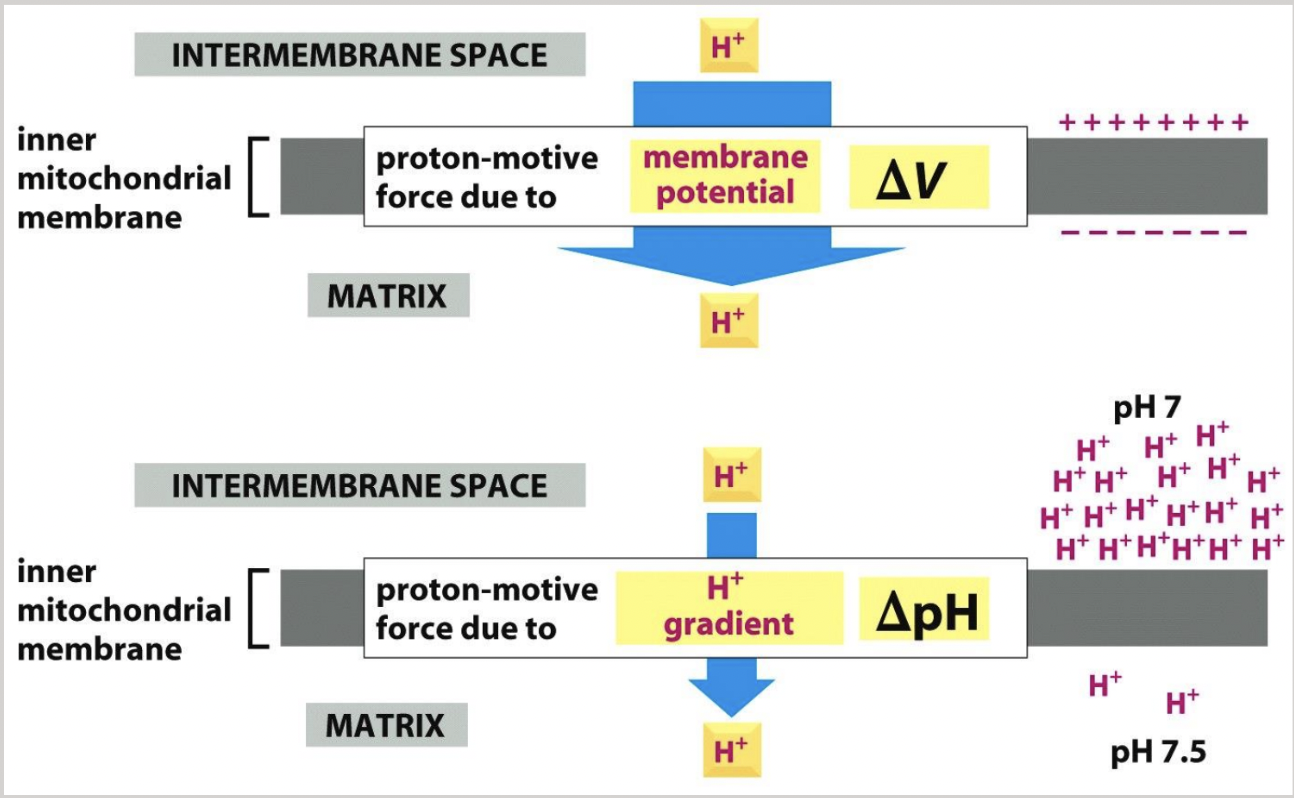
the net result is H+ pumping across the inner membrane (from the matrix to the intermembrane space)
driven by the energetically favorable flow of electrons
two consequences:
a pH gradient is generated
pH 7.5 in matrix, pH 7.0 in intermembrane space
a voltage gradient is generated across the inner mitochondrial membrane
negative within matrix, positive within intermembrane space
electrochemical proton gradient
energy generating metabolism summary
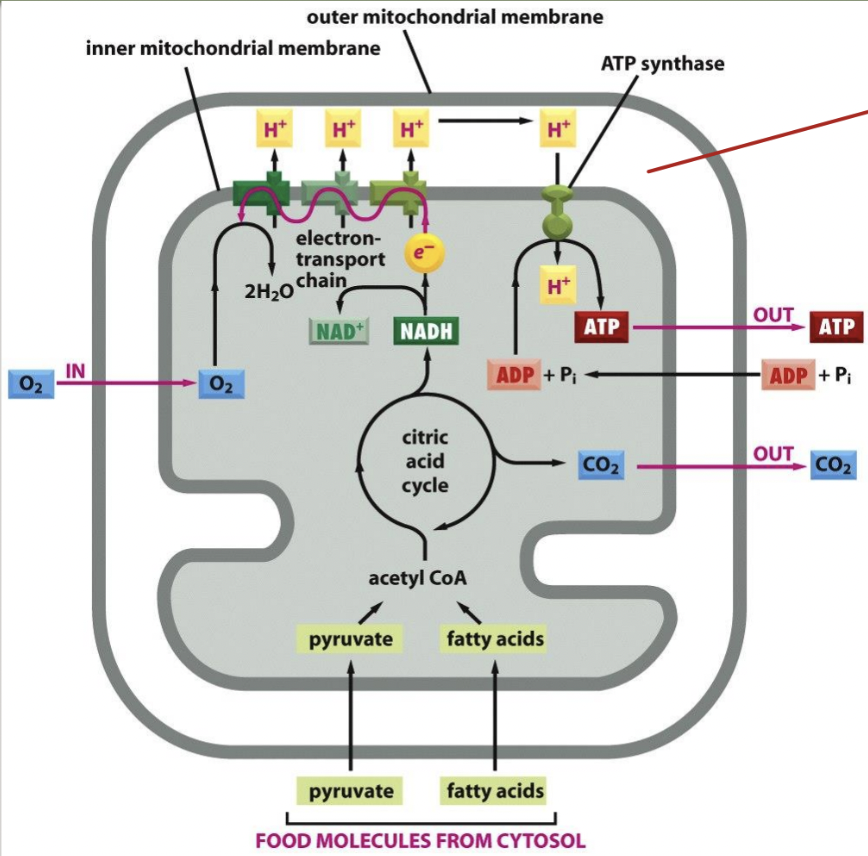
ATP synthesis
the electrochemical proton gradient across the inner mitochondrial membrane drives ATP synthesis
ATP synthesis is performed by the membrane-bound enzyme ATP synthase
creates a hydrophilic pathway across the inner membrane that allows H+ passage down the electrochemical gradient
can also reverse to hydrolyze ATP and pump H+
proton gradient transport
the electrochemical proton gradient drives several other processes:
transport of charged molecules into the matrix
pyruvate
inorganic phosphate
ADP and ATP are co-transported in opposite directions by a single transporter
ATP has one more negative charge than ADP, voltage differences across membrane drives transport
redox potential
measure of electron affinities
the tendency for redox reactions to move forward (for an electron to be removed from one molecule and added to the next) depends on the free energy change (deltaG) for the electron transfer
deltaG depends on the relative affinities of the two molecules for electrons
redox pairs: pairs of compounds that can transfer electrons
NADH ←> NAD+ + H+ +2e-
NADH is a strong electron donor, therefore NAD+ is a weak electron acceptor
can measure the redox potential of redox pairs
need an electrical circuit linking a 1:1 mixture of one redox pair to a second redox pair that can act as a reference standard
measure the voltage between them
voltage difference = redox potential
NADH/NAD+ = low redox potential
low affinity for electrons, good molecule for donating electrons
O2/H2O = high redox potential
high affinity for electrons, good molecule to act as a “sink” for electrons at the end of the pathway
electron transfers release energy
1:1 mixture of NADH/NAD+ has a redox potential of -320 mV
1:1 mixture of O2/H2O has a redox potential of +820 mV
transfer of an electron from NADH to O2 has a free energy change of deltaG0 = -26.2 kcal/mol
huge free energy drop means most energy would be lost as heat
stepwise process allows cells to store almost half of energy released
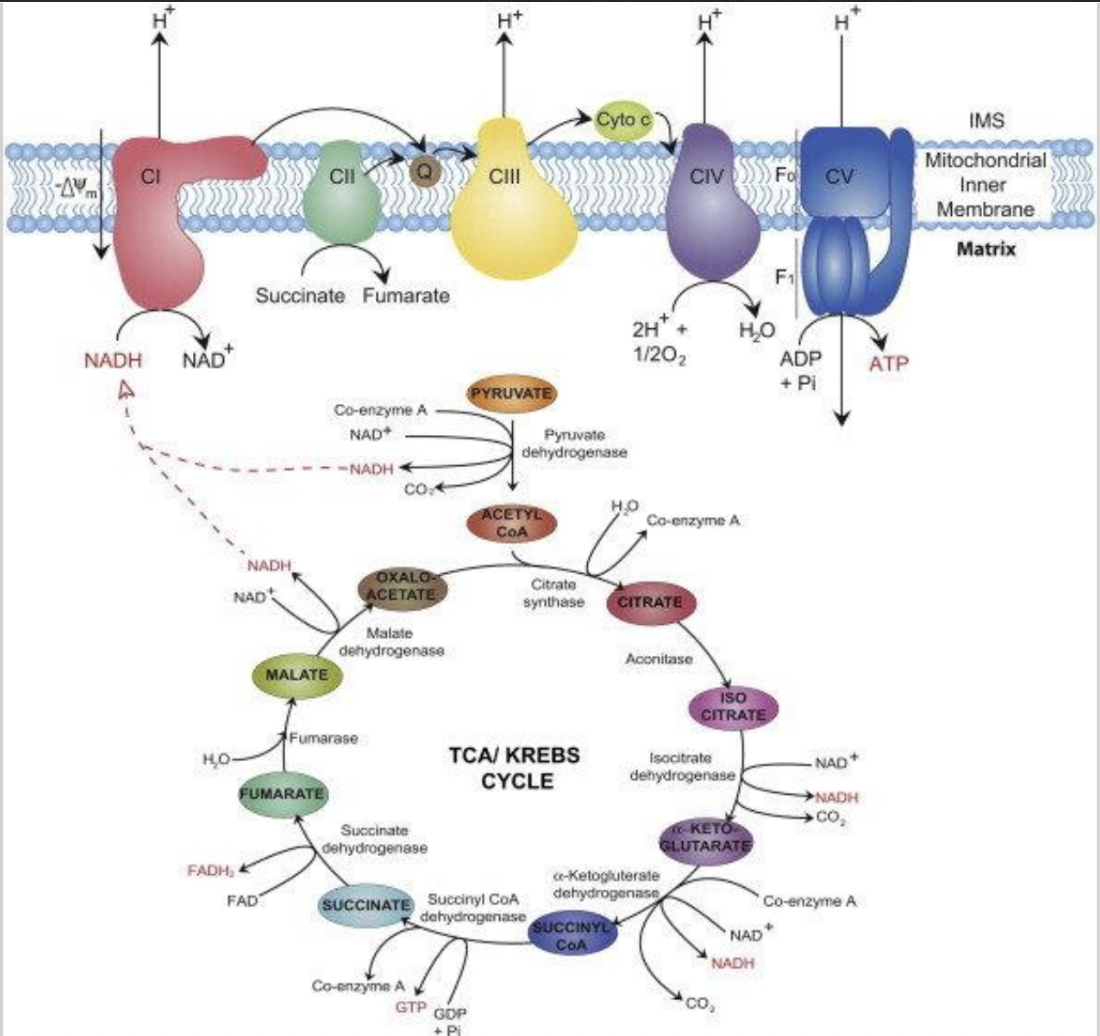
anaerobic metabolism
anaerobic respiration: production of energy in the absence of oxygen
alternative electron acceptor
nitrate
growth without using the ETC = fermenration
organic substrate undergoes a balanced series of oxidative and reductive reactions
end product formed in response to the cell’s need to balance consumption and production of reducing power
fermentation
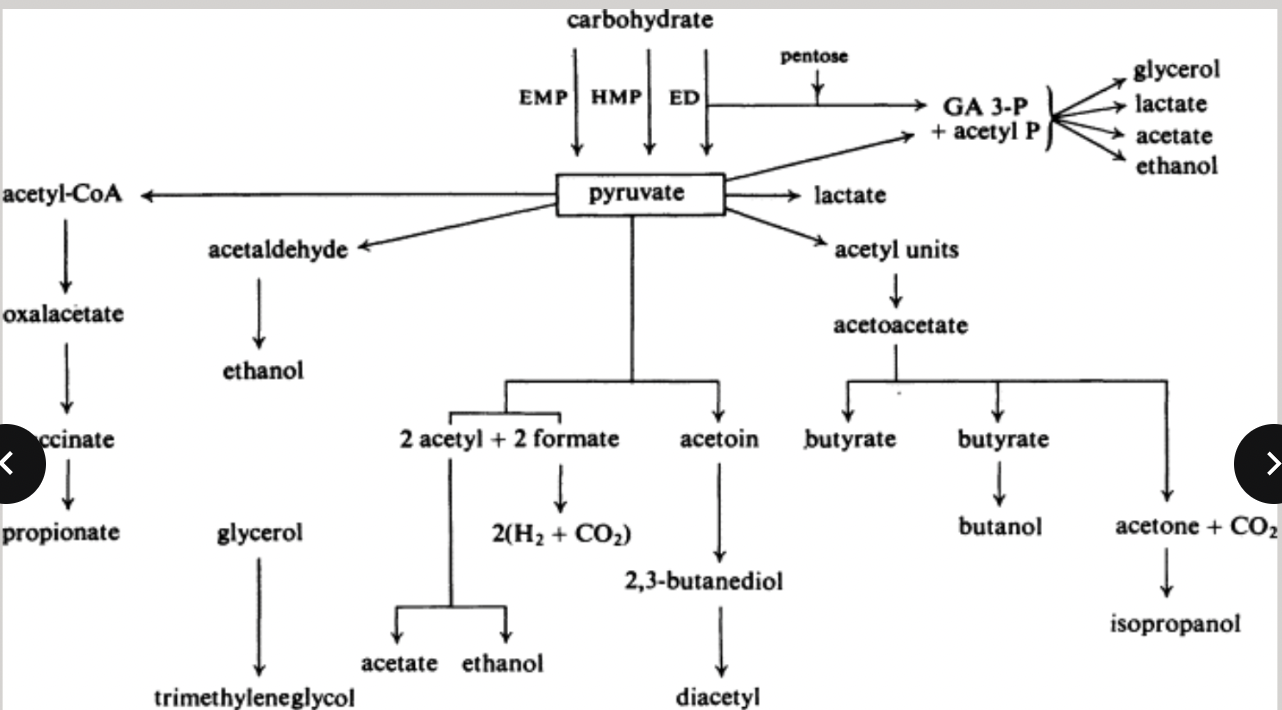
autotrophic metabolism
heterotrophic growth: organic molecules serve as carbon energy sources
autotrophic growth: energy for growth can be supplied by CO2
photoautotroph: light
chemoautotroph: oxidation of inorganic chemicals
aerobic and anaerobic metabolism
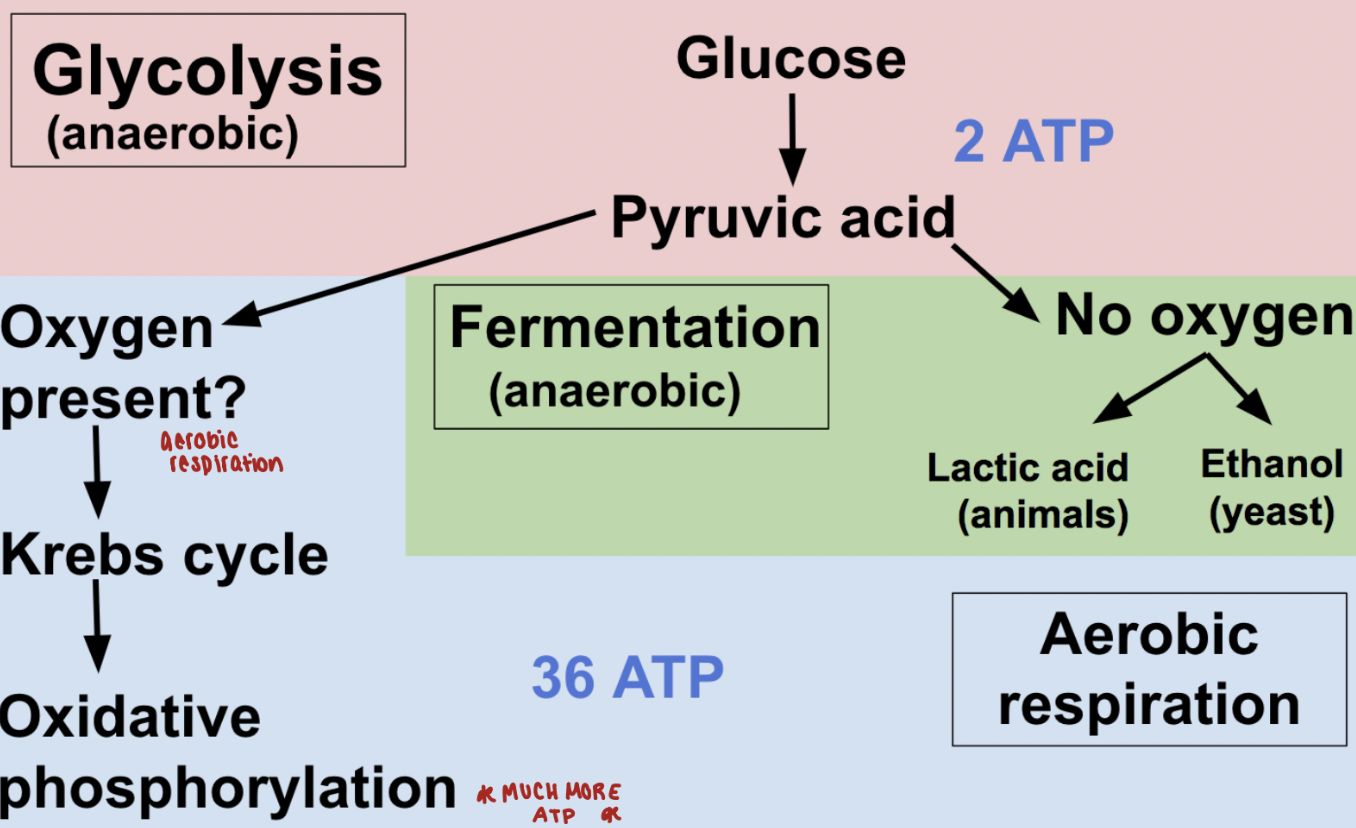
cell growth
