exam 2
1/111
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
112 Terms
axo-dendretic
majority of synapses in the brain
axon terminals synapse with dendrites or on dendretic spines
axo-axonic
synapses that allow for regulation of neurotransmitter release from the targeted axon terminal
input location & strength
distance between the synapse & axon hillock is inversely proportional to the ability
what influences the synapse more
axo-somatic synapses tend to influence the post synaptic neuron more than axo-dendretic synapses
neurotransmitter
a chemical, gas, or hormone that is synthesized in & released from a neuron
never go into the cell
large neurotransmitters
peptides and hormones
synthesized in the soma & then transported down to the axon terminal (microtubules)
small neurotransmitters
amino acids, monoamines, acetoycholine, & gases
synthesized in the terminals
vessicles
where neurotransmitters are stored in the membrane
steps to neurotransmission
action potential is porpogates over presynaptic membrane
depolarization of the terminal leads to Ca2+ influx
Ca2+ promotes exocytosis, the fusion of vesicles w/ membrane which releases neurotransmitter into cleft
bining of neurotransmitter to receptors open channels permitting ion flow
excitatory or inhibitory postsynaptic potentials spread over dendrites and the cell body to the axon hillock
what ends neurotransmission
transmitter degredation (enzymes)
transmitter removal through reuptake tansporters
autoreceptor activation (regulate calcium channels & machinery involved in exocytosis)
diffusion (neurotransmitters simply move out of the synaptic cleft
two mechanisms of neurotransmitter deactivation
reuptake: usually occurs with small neurotransmitters
enzyme degredation: breakdown thriugh enzymes usually accurs with large neurotransmitters
preventing neurotransmitter rerelease
autoreceptors
autoreceptors
receptors that are located on the edges of the active zone on the presynaptic terminal, regulate calcium channels and the machinery involved in exocytosis
released neurotransmitters are deactivated by either reuptake or enzymatic degredation
transmitter receptors
ionotropic receptors = ligand gated receptors
metabotropic receptors
metabotropic receptors II
ionotropic receptors
ligand-gated ion channel
fast transmission
neurotransmitter binds directly to the receptor
channel opens immediately
ions flow across the membrane for a brief time
metabotropic receptors
indirectly linked with ion channels through signal transduction mechanisms
G protein-coupled receptors (GPCR’s)
slower transmission
neurotransmitter binds to a GCPR
G protein activates
G protein binds to an adjacent ion channel
metabotropic receptors II
G protein-coupled receptors (GPCR’s)
slower transmission
neurotransmitter binds to a GCPR
G protein activates
G protein activates a second messenger
second messengers act on ion channels & other cellular targets
neurotransmission gap junctions
specialized type of synapse between 2 excitable cells (cardiac muscle cells, smooth muscle cells, & some neurons
ions flow directly from one neuron to another
0 time delay
no neurotransmitter required
good for synchronus activation of muscle fibers
signal versatility
any given neuron can release only a few types of neurotransmitters but can recieve & respond to multiple
receptor subtypes
the same neurotransmitter may bind to a variety of subtypes which trigger different responses (some excitatory, some inhibitory)
inhibitory effects of neurotransmitters
reduce the # of action potentials in the postsynaptic cell (usually through Cl- ligand gated channels)
excitatory effects of neurotransmitters
increase # of action potentials (usually not through Na+ channels)
ligand-receptor interactions
receptors only recognize certain ligands and this interaction is based mostly on the chemical/physical structure of the ligand
since neurotransmitters have different chemical structures, the interaction between a receptor and its transmitter is specific (only glutamate can activate glutamate receptors)
multiple receptors for each transmitter so one can activate different receptors
major families of neurotransmitters
amino acids; made in axon terminal
amines: made in axon terminal
peptides: made in soma
gases: made in axon terminal
amino acids
glutamate: small, ionotropic (excitatory), metabotropic (inhib, & exc), STOP
GABA: small, GABA-A (ionotropic, inhibitory), GABA-B (metabotropic, inhibitory), go
amines
dopamine: small, only metabotropic (inhib, & exc)
serotonin: small, 1 ionotropic (excitatory), metabotropic (in, & exc)
acetylcholine: small, nicotinic (ionotopic, excitatory), muscarinic (metabotropic, both)
norepinepherine: small, only metabotropic (inhibitory, & excitatory)
peptides
many (100’s): large, all kinds ionotropic (both), metabotropic (both)
gases
nitric: small, no receptors
roles of amino acids
small molecule neurotransmitters
building blocks of proteins
glutamate, glycine, aspartate (from food, have excitatory effects)
mediate majority of fast, directed synapses
signal is terminated through reuptake mechanisms
roles of amines
more diffuse effects than amino acid neurotransmitters
monoamines: dopamine, epinepherine, & norepinepherine are made from tyrosine
indolamines: serotonin made from trytophan (in milk & turkey)
signal terminated through reuptake & are broken down by enzymes
roles of pepties
slower signaling than small neurotransmitters
signal is terminated by degredative enzymes in synaptic cleft
released by cell body & dendrites
roles of soluble gases
nitric oxide & carbon monoxide
made in cell body & diffuse across membrane
believed to play a role in retrograde transmission (signaling from post neuron to presynaptic neuron)
NO & CO expand blood vessels to increase oxygen flow
short lived
broken down quickly by enzymes
psychoactive drugs
drugs that affect our behavior
must have a binding sit in the brain
must cross the blood brain barrier
drug action
molecular changes that can occur as a result of a drug or neurotransmitter binding to a receptor
drug effect
widespread physiological or psychological changes as a result of these molecular changes
a ligand
a subatance that binds to a receptor and has one of three effects
agonist
antagonist
inverse agonist
agonist
imitates the normal effects of the transmitter by copying the neurotransmitter
initiates the endogenous effects of the receptor
antagonist
blocks the receptor from being activated by other ligands
inverse agonist
initiates an effect that is the opposite of the normal function
indirect agonist
enchances activation of the post synaptic receptors by increasing the levels of the neurotransmoitter in the synaptic cleft
co-agonist
works to increase the ability of the neurotransmitter to bind tot he receptor - has a seperate binding site
competitive ligands
agonists, antagonists, or inverse agonists
because they bind to the same part of the receptor molecule as endogenous ligands
noncompetitve ligands
co-agonists, non-competitve antagonists
bind to secondary sites on the receptor seperate from the normal binding site of the receptor
binding affinity
the degree of chemical attraction between a ligand & a receptor
how strong a drug binds
efficacy
the ability of a bound ligand to activate the receptor
how likely a drug is to exert a biological response to the receptor
what are affinity and efficacy determined by
the chemical or structural properties of a compound (size, shape, charge, etc.)
what happens if a drug has a low affinity for a receptor
then the drug will quickly uncouple from the receptor to bind half the receptors at any given time a higher concentration of the drug is needed
what happens if a drug has a high affinity for a receptor
the two weill stay together for a longer period of time, and a lower concentration of drug will be bound to more receptor at any time
drug potency
a drug with a higher potency will evoke a larger response at a low concentration
a drug with a lower potency will evoke a smalle rrsponse at a low concentration
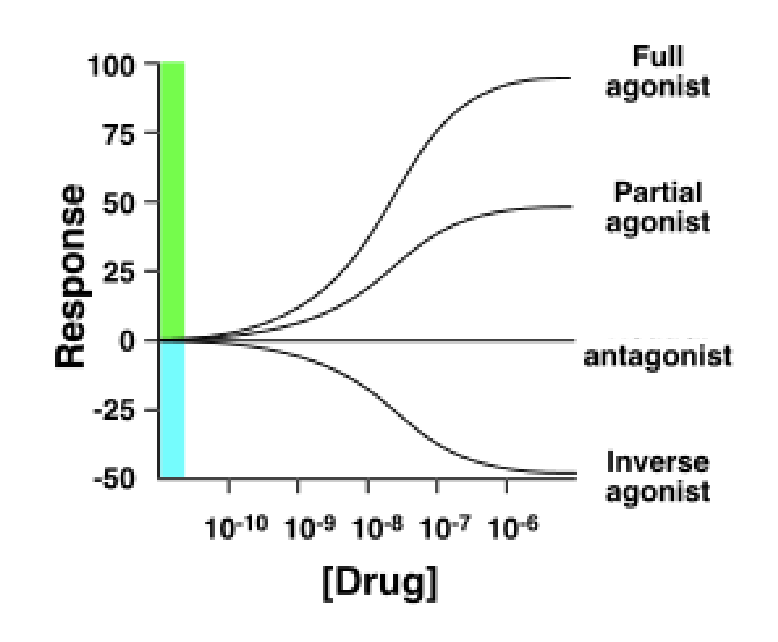
done response curve
a graph of the relationship between drug doses and the effects
a tool to understand pharmacodynamics (the functional relationshio between drugs & their targets)
measures of efficacy
agonists have high efficacy
antagonists have low efficcy but have affinity
partial agonists have a medium response regardless of dose
full inverse agonists have high efficacy
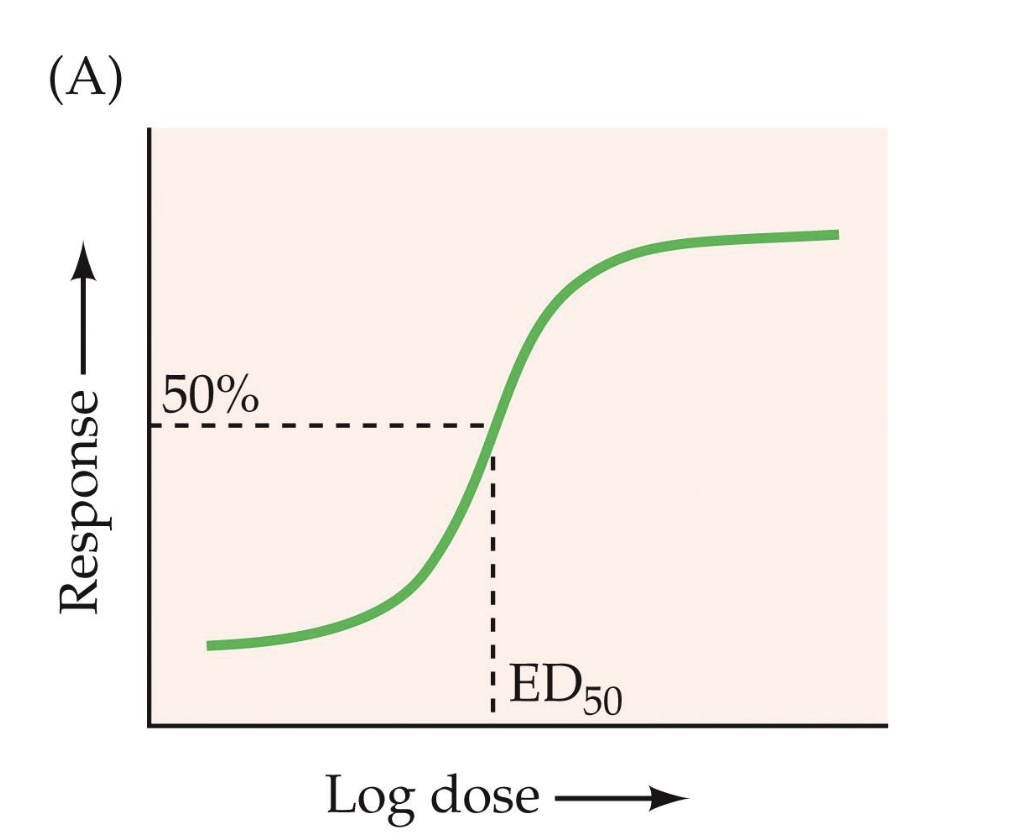
ED50
the dose at which the drug shows half of its maximal effect “the effective dose”
the dose at which the drug procuses a given effect in 50% of the population
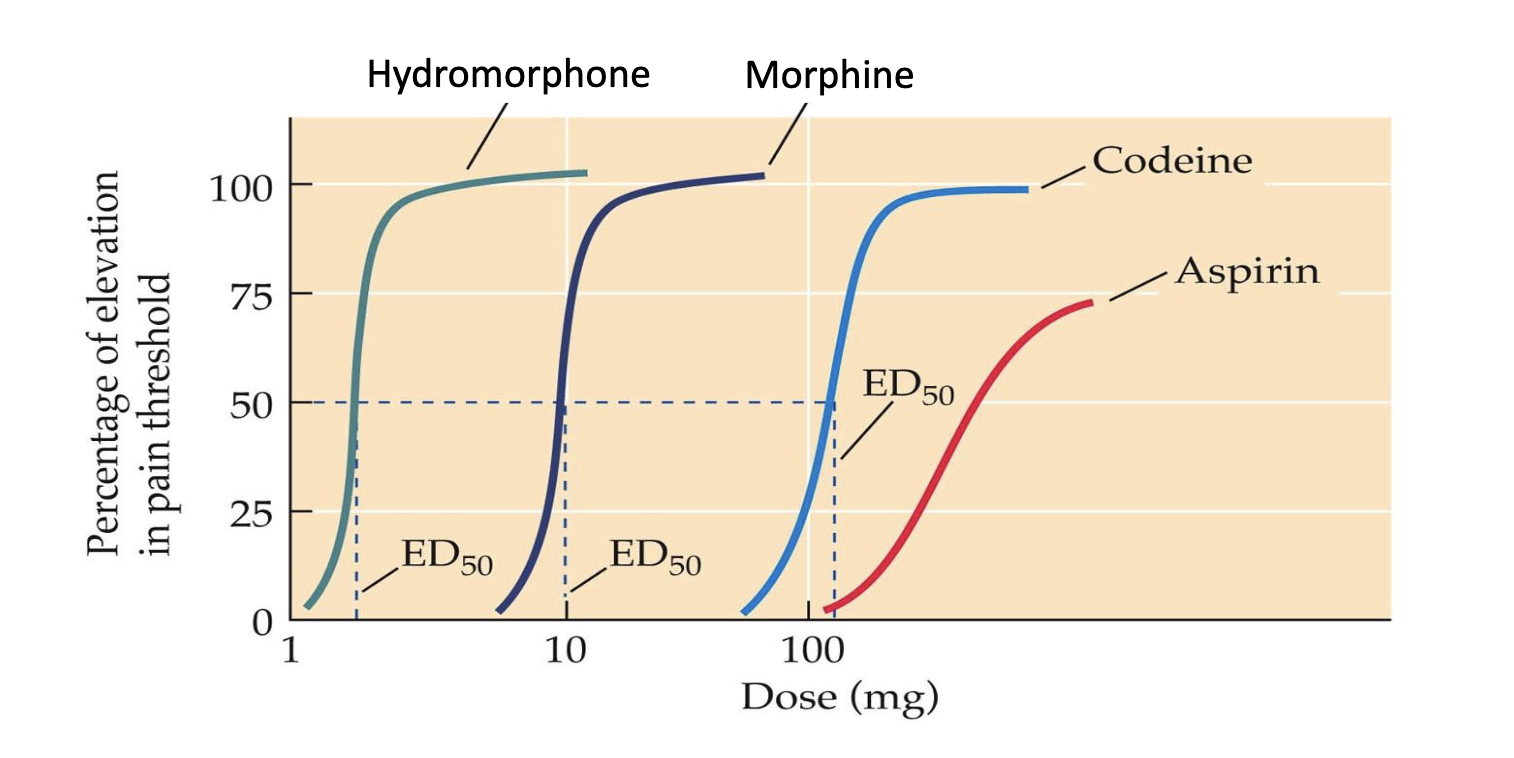
relative potency
the relative potencies of the drug by comparing their ED50 values
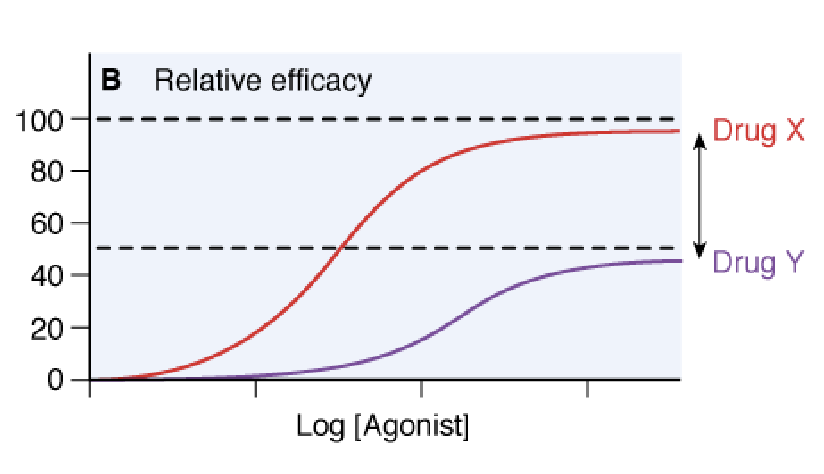
relative efficacy
the ability of a drug to produce a response
TD50
median toxic dose, dose required to get 50% of the population to report a specific toxic effect
LD50
median lethal dose, dose required to reach 50% mortality
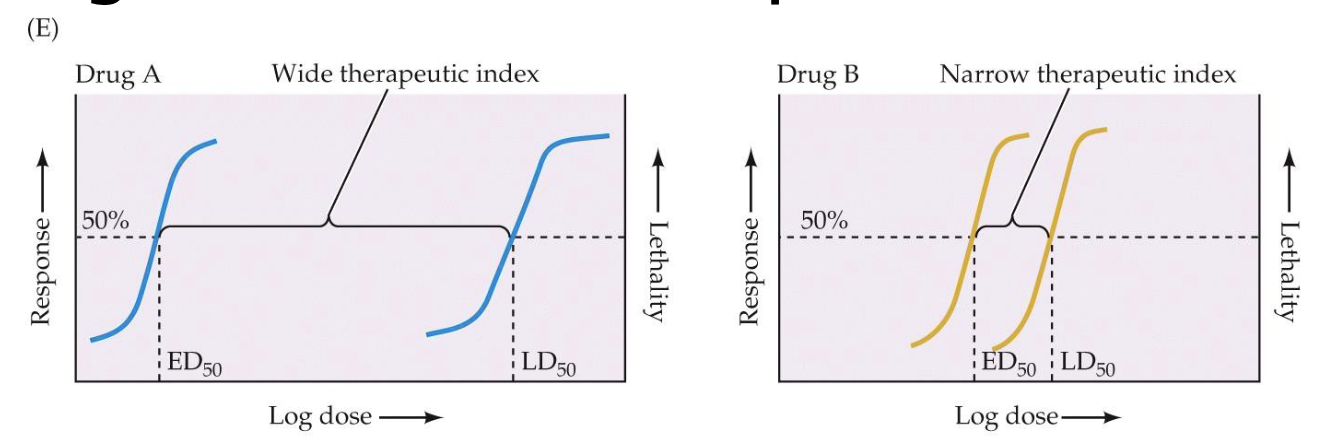
theraputic index
the gap between the effective dosage of a drug and the toxic dosage
Compares the ED50 to the TD50 or LD50
routes of administration
ingestion/oral
absorption
inhalation
injection
ingestion/oral
‘first pass' metabolism by liver & gut walls
absorption
bypass the liver no ‘first pass’ metabolism
sublingual: under tongue → blood → brain
cutaneous: on skin → slow or fast release depending on fattiness
rectum: → blood → brain
inhalation
bypass the liver
nose or lungs: → into blood → into brain
injection
bypass the liver
intravenous: → into blood → into brain
subcutaneous: under skin → into fat → slow release
intramuscular: into muscle → slow release
drug circulation
depends on the route of administration
drug distribution: how soluble the drug molecule is in fat (ability to pass membrane, ability to bind to blood proteins)
drug metabolism: enzymes convert drugs into metabolites, depending on the drug these can be harmful, therapeutic, or inactive
drug elimination: through the kidneys or by enzymes in the liver
drug distribution
how soluble the drug molecule is in fat
ability to bind to blood proteins
drug metabolism
enzymes convert drugs into metabolites
depending on the drug these may be theraputic, harmful, or inactive
drug elimination
depends on how its administered
oral drugs are excreted into urine via the kidneys and/or by inactivation by enzymes in the liver
drug penetration of CNS
blood brain barrier
mechanisms of drug actions
diffusely
specifically
tolerance & addiction
with repeated use, the desired effect requires larger doses
decrease in response elicited by the same dose
increase in the amount needed to get the same effect
withdrawl symptoms
discomfort or distress that follow discontinuing drug
physical sign of dependence
tolerance and withdrawl
manifestations of the same underlying physiological change
cross-tolerance
tolerance to a whole class of chemically similar drugs
sensitization
occurs when drug effects become stronger with repeated use
metabolic tolerance
metabolic systems in the body (eg. liver) become more effective in eliminating the drug before it reaches the brain
functional tolerance
target issue/cells show less sensitivity to drug
receptor desensitization
after prolonged/chronic agonist activation, receptors can decrease in number
receptor sensitization
after prolonged/chronic abscence of agonist activation, receptors can increase in number
adaptations in postsynaptic receptors
when there are abnormailites in NT levels
when administering drug repeatedly
hyperstimulation
hypostimulation
hyperstimulation
too much NT’s
fewer receptors, reduced function
reduce signal to postsynaptic neuron
hypostimulation
too little NTs
more receptors, enhanced function
enhanced signal in postsynaptic neuron
contingent drug tolerance
tolerance only develops to drug effects that are experienced
conditioned drug tolerance
tolerance expressed only in the prescence of drug predictive stimuli
if you’re always taking drugs after certain cues you develop a certain tolerance to the drugs effects in preparation for taking that drug
context-dependent studies
tolerance in one environment but if you take it in another environment it might have more of an effect because you don’t have a tolerance there
novel environments lead to drug overdose
incubation of drug cravings
cues can trigger drug craving and relapse
cues presented soon after drug withdrawal have less of an effect than cues presented later
liking (reward pathway)
pleasure, euphoria
conscious
GABA, opiods, dopamine
ventral palladium, paraventricular, nucleus accumbens
taking drugs sometimes
dips for the most part
wanting (reinforcement pathway)
makes you seek out the drug
craving, appetite, drive
mostly unconscious (like being hungry)
dopamine
nucleus accumbens, ventral tegmental area, amygdala
seeing or taking drugs
keeps increasing
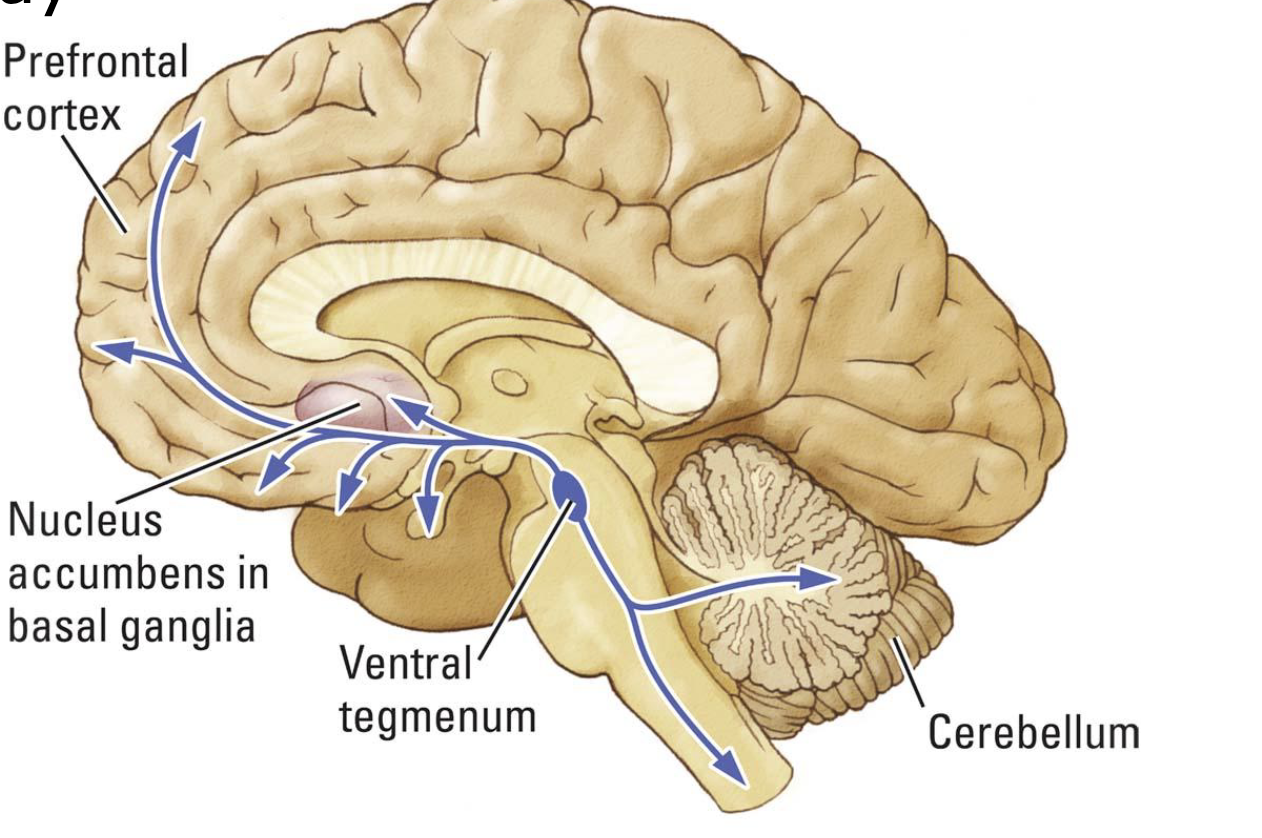
mesocorticolimbic pathway
nearly all drugs of abuse work to increase dopamine in the nucleus accumbens
natural reinforcers like food, sex, and exercise also do this
nucleus accumbens and addiction
critical to reward and “wanting”
self-administration of addictive drugs
leads to conditioned place-preferences
natural reinforcers
three stages of addiction development
initial drug use
habitual drug taking
positive incentive value - expectations
wanting vs. liking
incentive-sensitization
dopamine in the nucleus accumbens
drug craving and addiction relapse
stress
drug priming (the effect you’re expecting to get)
conditioned environmental cues
severity of substance abuse disorder
mild: 2-3 symptoms
moderate: 4-5 symptoms
severe: 6+ symptoms
habitual drug users who continue to use depite adverse effect and repeated attempts to stop
addiction =/= physical dependence
drug effects on conduction
local anesthetics can block Na+ channels, preventing action potentials
drug effects on synaptic transmission
disrupt storage of NT into vessicles
disrupt release of NT - exocytosis
alter NT reuptake - interference with transporters
alter NT degredation - interfere with enzymes in the synapse
stimulants
increase feelings of energy and well-being, produce sympathetic nervous system effects (fight or flight)
cocaine, amphetamines
nicotine, caffine, pseudopherine (allergy meds)
amphetamines & methamphetamines
injected, inhaled
reverses dopamine and norepinepherine transporters (indirect agonist) (instead of sucking up NTs they spit them out)
powerfully addictive
greater risk of developing parkinsons
cardiographic abnormalities
coacine/crack
from coca leaf
commonly inhaled, absorbed across mucosal membranes, or injected
blocks catecholamine (dopamine, norepinepherine, & serotonin) reuptake transporters (indirect agonist)
euphoria (effects of dopaminergenic neurotransmission)
crash of agitated depression within 15 to 30 minutes after neurotransmitters drop
cocaine sprees
large doses can cause psychosos with schizophrenic effects
nicotine
from tobacco leaf
usually inhaled
agonist at nicotinic (ionotropic) acetylcholine receptors
very fatty so it readily crosses the BBB
heritability 55%
causes compulsive drug cravings and withdrawl symptoms which contributes to relapse
70% change of becoming addicted
reaches brain in 7 seconds
caffine
orally consumed
antagonist of select adenosine receptors found on GABA receptors
inhibits GABA release
antagonists of adenosine receptors on presynaptic glutamate neurons
autoreceptors
bind to neurons and are sensitive to the neurotransmitters released only by the neuron they are attached to
when they feel like that neurotransmitters levels are getting too high it tells the neuron to stop releasing that neurotransmitter causing a negative feedback loop
heteroreceptors
bind to neurons and are sensitive to the neurotransmitters released by the adjacent different neuron they are attached to
when they feel like that neurotransmitters levels are getting too high it tells the neuron to stop releasing that neurotransmitter causing a negative feedback loop