Alkane Nomenclature
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
1 carbon
methane
2 carbons
ethane
3 carbons
propane
4 carbons
butane
5 carbons
pentane
6 carbons
hexane
7 carbons
heptane
8 carbons
octane
9 carbons
nonane
10 carbons
decane
straight chain substituents
use carbon chain prefix but add “-yl” at the end instead of “ane” (i.e. ethyl instead of ethane)
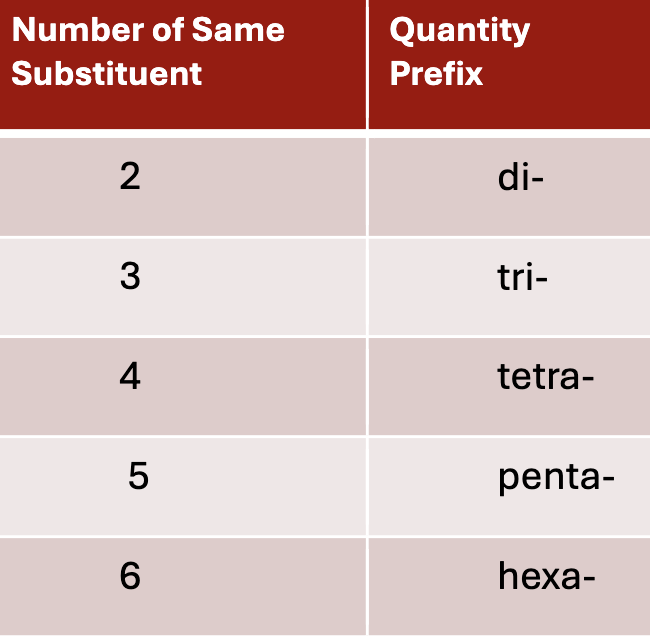
naming when multiple of the same substituents are present
naming cyclic molecules by themselves
based on number of carbons in the ring-
naming cyclic molecules with substituents shorter than the cyclic ring
treat ring as main carbon chain
naming cyclic compounds when a carbon chain is longer than the cyclic compound
treat the cyclic structure as a substituent
cis and trans classification
ONLY FOR DISUBSTITUTED CYCLIC ALKANES
if the 2 substituents are on the same plane (wedge or dash) it’s cis
if the 2 substituents are on different planes (wedge and dash) it’s trans
finding the longest carbon chain for naming halides
has to be the longest carbon chain that has the halide on it
how do we treat halogens
with kindness (like a substituent)
F
fluoro
Cl
chloro
Br
Bromo
I
Iodo
Primary carbon
CH3
Secondary Carbon
CH2
Tertiary Carbon
CH
Defining primary/secondary/tertiary carbon
Number of non-H groups bonded to the carbon
Alkyl Halide Classification
Number of groups on the alpha carbon