Covalent bonds
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Where does covalent bonding occur?
Between 2 non-metals
What is a covalent bond ?
The strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
Name and describe the two exceptions to the octet with examples
There are not enough electrons to fill the octet - some compounds have less than 8 electrons in their outer shell , like Boron Triflouride (BF3) , which has 8 electrons in each outer shell of fluorine , but only 6 outer electrons in Boron
Expand the octet - a few compounds can use d orbitals to have more than 8 electrons in their outer shell . In Sulfur Hexaflouride , sulfur has 12 electrons in its outer shell and each fluorine has 8 electrons in its outer shell
Why do some atoms share more than one pair of electrons and what are examples ?
Double or triple bonding occurs between some elements , like oxygen for a double bond and nitrogen can triple bond
What is dative covalent bonding and an example ?
Where both electrons come from one atom .
E.g : In an ammonium ion (NH4 ) + , the nitrogen atom donates a pair of electrons to a proton (H+)
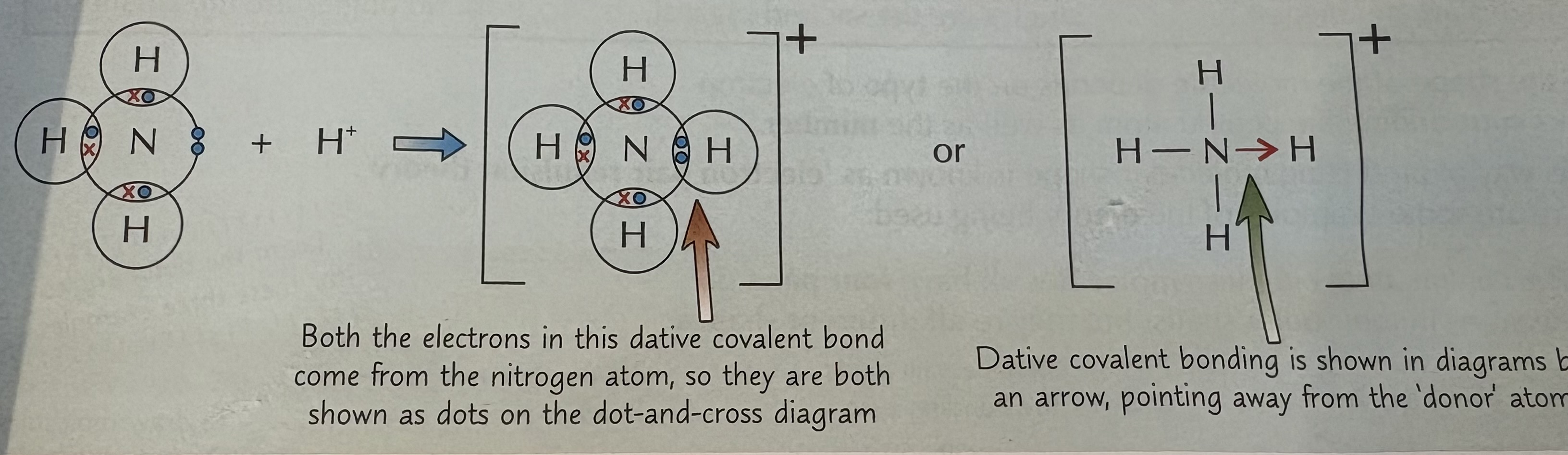
How is the strength of a covalent bond measured ?
The average bond enthalpy measures the energy required to break a covalent bond
The stronger the bond is , the more energy required to break it , and so the greater value of the average bond enthalpy