atomic structure
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
relative mass & relative charge of proton
- 1
- +1
relative mass & relative charge of neutron
- 1
- 0
relative mass & relative charge of electron
- 1/2000
- -1
what is the mass number
- top number
- total number of protons & neutrons in the nucleus
what is the atomic number?
- bottom number
- proton number
what is an isotope?
atoms of the same element with the same number of protons but different number of neutrons
what are ions
ions have a different number of electrons and protons
what does the number & arrangement of electrons decide?
the chemical properties of an element
what did dalton describe atoms as?
solid spheres
what did thompson describe atoms as?
plum-pudding model; ball of +ve charge with -ve electrons embedded throughout
what was rutherford's experiment?
alpha scattering experiment
fired alpha particles at a thin layer of gold foil
most of the alpha particles passed straight through the foil
some were deflected backwards (they must have hit a small positive nucleus)
showed how atom was mostly empty space, with a tiny, positively charged nucleus at the centre
what did bohr describe atoms as?
- tiny, positively charged nucleus at the centre
- electrons in shells
- each shell has a fixed energy
- when electrons move between shells, they emit or absorb electromagnetic radiation
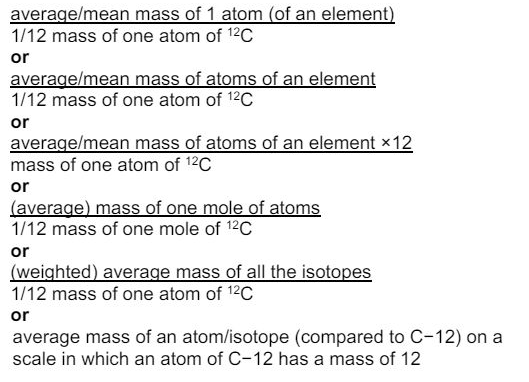
what is the relative atomic mass of an atom?
- the average mass of an atom of an element compared to the mass of 1/12th of a carbon-12 atom
what is the relative isotopic mass?
the mass of an atom of an isotope of an element where an atom of carbon-12 is exactly 12
how to calculate relative atomic mass
(mass 1 x abundance 1) + (mass 2 x abundance 2)/ total abundance
what is the relative molecular mass?
the average mass of a molecule compared to 1/12th of the mass of a carbon-12 atom
what is the relative formula mass?
the average mass of a formula unit on a scale where the mass of a carbon-12 atom is exactly 12
what is mass spectrometry?
a powerful instrumental technique used to find the relative mass of elements & compounds
what is a condition of mass spectrometry?
- vacuum: to prevent the ions produced colliding with molecules from the air
what are the steps of mass spectrometry?
- ionisation
- acceleration
- ion drift
- detection
what are the 2 types of ionisation in a mass spectrometer?
- electrospray ionisation
- electron impact ionisation
what is electrospray ionisation?
- sample is dissolved in solvent
- pushed through a small nozzle at high pressure
- high voltage is applied to it, causing each particle to gain a H+ ion
- solvent is removed, leaving a gas made of positive ions
what is electron impact ionisation in a mass spectrometer?
- sample is vaporised
- electron gun is used to fire high energy electrons at it
- this knocks one electron off each particle, so they become +1 ions
what happens during acceleration in the mass spectrometer?
- positive ions are accelerated by an electric field
- the electric field gives the same kinetic energy to all ions
- lighter ions experience a greater acceleration
what happens during ion drift in a mass spectrometer?
- the ions enter a region with no electric field
- they drift through this at the same speed as they left the electric field
- lighter ions drift at higher speeds
what happens during detection in a mass spectrometer?
- lighter ions travel through the drift region at higher speeds, so they reach the detector quicker
- detector detects the current created when ions hit it & records how long they took to pass through the spectrometer
what is the data produced by the detector used for?
- used to calculate the mass/charge values needed to produce a mass spectrum
how to work out relative atomic mass?

what are the 4 types of electron shell sub-shells?
- s
- p
- d
- f
what is another word for sub-levels?
orbitals
which orbitals are in principle quantum level 1?
1s
which orbitals are in principle quantum level 2?
2s2p
which orbitals are in principle quantum level 4?
4s4p4d4f
which orbitals are in principle quantum level 3?
3s3p3d
what i’ve learnt so far:
principle quantum= energy level
number of electrons in shell= formula is 2n² e.g 2(4)² = 32 so each energy level can hold 2,8,18,32
energy levels fill in as 2,8,8,2 then fill in from the 3rd level then 4th
sometime labelled as K,L,M,N
highest energy electron= valence electron= the outermost electron (available for reactions and bond formation)
subshells= s,p,d,f
n subshells inside
1 s
2 s,p
3 s,p,d
4 s,p,d,f
subshells number of orbitals
s 1
p 3
d 5
f 7
each orbital can hold upto 2 electrons so 2,6,10,14
rows going across from left to right are periods all these elements have the same number of energy shells as we go down the table, the number of energy shells increase
columns going down from top to bottom are called groups all these elements have the same number of valence electrons. This is why elements in the same group have similar properties
old system for the periodic table has only 1-8 groups which also means each element in that group has the same number of valence electrons
but the new system is that the groups are 1-18 including the d-block
to find out the number of valence electrons in each group=
1and2= group number is the same as valence electrons
13-18= subtract 10 from the group number
d orbital= electron config? The maximum valence electrons in the d-block is 2 (from the s orbital) + up to 10 (from the d orbital) = 12.
My analogy to understand the structure:
Hotels = Energy Levels (n) → (Main energy levels where electrons exist)
Floors = Subshells (s, p, d, f) → (Types of subshells in each energy level)
People = Orbitals → (Each orbital is a "person" in a room)
Pearls = Electrons → (Each "person" can hold 2 pearls, meaning 2 electrons per orbital)
And the rules:
s rooms → 1 person (1 orbital)
p rooms → 3 people (3 orbitals)
d rooms → 5 people (5 orbitals)
Each person can only hold 2 pearls (electrons)!
why does the d block have s orbitals and d orbitals if its called the d block?
We call them the “d block” because the electron that distinguishes one element from another in these metals is being added to a d orbital, not the s orbital.
✔ Transition metals have both s and d orbitals because electrons fill the s orbital first before moving to d.
✔ The d orbital is higher in energy than the s orbital in the same period, even though its principal quantum number (n-1) is lower.
✔ When asked about the highest-energy electron in transition metals, the answer is always the d orbital.
Why Can’t We Say the Same Thing About p Orbitals?
Key Difference Between p and d Orbitals:
p orbitals are in the same energy level as s orbitals.
Example: In n = 3, you have 3s, 3p, and 3d.
They all belong to the same shell (n = 3), but p is higher in energy than s, and d is higher than p.
The filling order is: 3s → 3p → 3d (in increasing energy).
d orbitals belong to a lower principal energy level than the s orbital that fills before them.
Example: In period 4, the filling order is:
4s fills before 3d (even though 3d belongs to a lower principal energy level).
The reason? 4s is lower in energy than 3d, so it gets filled first.
So, while p orbitals are just a higher-energy part of the same energy level, d orbitals actually come from a lower energy level but have higher energy than the s orbitals of the next level.
So, even though 3d belongs to the 3rd level, electrons prefer to fill the 4s first because it’s more stable (lower energy). But once electrons do go into the 3d, they stay there because 3d has higher energy than 4s.
Now, if you’re asked:
❓ Which orbital is the highest-energy electron of transition metals found in?
✅ The answer is the d orbital because:
Even though s orbitals fill first, they have lower energy than d orbitals.
When looking for the highest-energy electron, we check the d orbitals in transition metals.
✔ s orbitals always fill first, even before p and d orbitals.
✔ p orbitals are just a higher-energy part of the same shell, but d orbitals are trickier because they belong to a lower shell but have higher energy.
✔ d orbitals have higher energy than s orbitals in the same period, which is why the highest-energy electron in transition metals is found in the d orbital.
👉 d-block elements include s orbitals because their highest-energy electrons are in d orbitals, but s electrons are still there.
👉 p-block elements don’t "include" s orbitals because their highest-energy electrons are in p orbitals, and the s orbital was filled in a lower energy level.
Why This Happens (Step-by-Step Explanation)
1⃣ s orbitals always fill first in any energy level.
Example: In period 3, the filling order is 3s → 3p.
2⃣ In the p-block, the s orbital is fully filled and “forgotten” because p orbitals come right after in the same energy level.
Example: Sulfur (S, Group 16)
Electron configuration: [Ne] 3s² 3p⁴
The highest-energy electrons are in 3p, so we call sulfur a p-block element, and we don’t talk about its 3s orbital anymore.
3⃣ In the d-block, the s orbital (ns) fills before the d orbital (n-1)d- (energy level before se.g. 3d), but both are still relevant.
Example: Iron (Fe, Group 8, d-block)
Electron configuration: [Ar] 4s² 3d⁶
Even though 4s fills first, the highest-energy electrons are in 3d.
Since both 4s and 3d are involved, we include the s orbital when talking about d-block elements.
Why Does the s Orbital Seem "Half-Filled" in d-Block Elements?
You might have noticed that some d-block elements only have one s electron instead of two (e.g., Cr → [Ar] 4s¹ 3d⁵).
This happens because:
✅ A half-filled (d⁵) or fully filled (d¹⁰) d orbital is more stable than having a full s orbital.
✅ Electrons can "move" from the s orbital into the d orbital to create a more stable configuration.
Example: Chromium (Cr, atomic number 24)
Expected: [Ar] 4s² 3d⁴
Actual: [Ar] 4s¹ 3d⁵ (because a half-filled 3d⁵ is extra stable).
But not all d-block elements do this! Many still have two s electrons, like iron (Fe) → [Ar] 4s² 3d⁶.
Final Takeaway (Short and Simple)
✅ p-block elements don’t “include” s orbitals because the p orbitals take over in the same energy level.
✅ d-block elements include s orbitals because the s orbital (ns) fills before the d orbital (n-1)d, and both remain relevant.
✅ Sometimes the s orbital only has one electron instead of two because the d orbitals become more stable when half-filled or fully filled.
![<p>principle quantum= energy level</p><p>number of electrons in shell= formula is 2n² e.g 2(4)² = 32 so each energy level can hold<strong> 2,8,18,32</strong></p><p>energy levels fill in as<strong> 2,8,8,2 </strong>then fill in from the 3rd level then 4th</p><p>sometime labelled as K,L,M,N</p><p>highest energy electron= <strong>valence electron</strong>= the <strong>outermost </strong>electron (available for reactions and bond formation)</p><p><strong>subshells</strong>= <strong>s,p,d,f</strong></p><p>n subshells inside</p><p><strong>1 s</strong></p><p><strong>2 s,p</strong></p><p><strong>3 s,p,d</strong></p><p><strong>4 s,p,d,f</strong></p><p>subshells number of orbitals</p><p><strong>s 1</strong></p><p><strong>p 3</strong></p><p><strong>d 5</strong></p><p><strong>f 7</strong></p><p>each orbital can hold upto 2 electrons so<strong> 2,6,10,14</strong></p><p></p><p><strong>rows </strong>going across from<strong> left to right</strong> are <strong>periods </strong>all these elements have the same number of energy shells as we go <strong>down </strong>the table, the number of<strong> energy shells</strong> <strong>increase</strong></p><p></p><p><strong>columns </strong>going down from top to bottom are called <strong>groups </strong>all these elements have the same number of <strong>valence electrons</strong>. This is why elements in the same group have similar properties</p><p>old system for the periodic table has only 1-8 groups which also means each element in that group has the same number of valence electrons</p><p>but the new system is that the groups are 1-18 including the d-block</p><p>to find out the number of valence electrons in each group=</p><p>1and2= group number is the same as valence electrons</p><p>13-18= subtract 10 from the group number</p><p>d orbital= electron config? The maximum valence electrons in the d-block is 2 (from the s orbital) + up to 10 (from the d orbital) = 12.</p><p></p><p>My analogy to understand the structure:</p><p></p><ol><li><p><strong>Hotels = Energy Levels (n)</strong> → (Main energy levels where electrons exist)</p></li><li><p><strong>Floors = Subshells (s, p, d, f)</strong> → (Types of subshells in each energy level)</p></li><li><p><strong>People = Orbitals</strong> → (Each orbital is a "person" in a room)</p></li><li><p><strong>Pearls = Electrons</strong> → (Each "person" can hold 2 pearls, meaning 2 electrons per orbital)</p></li></ol><p>And the <strong>rules</strong>:</p><ul><li><p><strong>s rooms → 1 person (1 orbital)</strong></p></li><li><p><strong>p rooms → 3 people (3 orbitals)</strong></p></li><li><p><strong>d rooms → 5 people (5 orbitals)</strong></p></li><li><p><strong>Each person can only hold 2 pearls (electrons)!</strong></p></li></ul><p></p><p></p><p><strong>why does the d block have s orbitals and d orbitals if its called the d block?</strong></p><p>We call them the “d block” because the electron that distinguishes one element from another in these metals is <strong>being added to a d orbital, not the s orbital.</strong></p><p><span data-name="check_mark" data-type="emoji">✔</span> Transition metals have both s and d orbitals because electrons fill the s orbital first before moving to d.<br><span data-name="check_mark" data-type="emoji">✔</span> The d orbital is higher in energy than the s orbital in the same period, even though its principal quantum number (n-1) is lower.<br><span data-name="check_mark" data-type="emoji">✔</span> When asked about the highest-energy electron in transition metals, the answer is always the d orbital.</p><p></p><p>Why Can’t We Say the Same Thing About p Orbitals?</p><p>Key Difference Between p and d Orbitals:</p><ul><li><p>p orbitals are in the same energy level as s orbitals.</p><ul><li><p>Example: In n = 3, you have 3s, 3p, and 3d.</p></li><li><p>They all belong to the same shell (n = 3), but p is higher in energy than s, and d is higher than p.</p></li><li><p>The filling order is: 3s → 3p → 3d (in increasing energy).</p></li></ul></li><li><p>d orbitals belong to a lower principal energy level than the s orbital that fills before them.</p><ul><li><p>Example: In period 4, the filling order is:</p><ul><li><p><strong>4s fills before 3d (even though 3d belongs to a lower principal energy level)</strong>.</p></li><li><p>The reason? <strong>4s is lower in energy </strong>than 3d, so it gets filled first.</p></li></ul></li></ul></li></ul><p>So, while p orbitals are just a higher-energy part of the same energy level, <strong>d orbitals actually come from a lower energy level but have higher energy than the s orbitals </strong>of the next level.</p><p></p><p></p><p>So, even though <strong>3d belongs to the 3rd level</strong>, electrons <strong>prefer to fill the 4s first</strong> because it’s <strong>more stable</strong> (lower energy). But once electrons do go into the 3d, they <strong>stay there because 3d has higher energy than 4s</strong>.</p><p></p><p>Now, if you’re asked:<br><span data-name="question" data-type="emoji">❓</span> <em>Which orbital is the highest-energy electron of transition metals found in?</em></p><p><span data-name="check_mark_button" data-type="emoji">✅</span> The answer is <strong>the d orbital</strong> because:</p><ul><li><p>Even though <strong>s orbitals fill first</strong>, they have <strong>lower energy than d orbitals</strong>.</p></li><li><p>When looking for the <strong>highest-energy electron</strong>, we check the <strong>d orbitals</strong> in transition metals.</p></li></ul><p></p><p><span data-name="check_mark" data-type="emoji">✔</span> s orbitals always fill first, even before p and d orbitals.<br><span data-name="check_mark" data-type="emoji">✔</span> p orbitals are just a higher-energy part of the same shell, but d orbitals are trickier because they belong to a lower shell but have higher energy.<br><span data-name="check_mark" data-type="emoji">✔</span> d orbitals have higher energy than s orbitals in the same period, which is why the highest-energy electron in transition metals is found in the d orbital.</p><p></p><p><span data-name="point_right" data-type="emoji">👉</span> d-block elements include s orbitals because their highest-energy electrons are in d orbitals, but s electrons are still there.<br><span data-name="point_right" data-type="emoji">👉</span> <strong>p-block elements don’t "include" s orbitals because their highest-energy electrons are in p orbitals, and the s orbital was filled in a lower energy level.</strong></p><p></p><p>Why This Happens (Step-by-Step Explanation)</p><p><span data-name="one" data-type="emoji">1⃣</span> s orbitals always fill first in any energy level.</p><ul><li><p>Example: In period 3, the filling order is 3s → 3p.</p></li></ul><p><span data-name="two" data-type="emoji">2⃣</span> In the p-block, the s orbital is fully filled and “forgotten” because p orbitals come right after in the same energy level.</p><ul><li><p>Example: Sulfur (S, Group 16)</p><ul><li><p>Electron configuration: [Ne] 3s² 3p⁴</p></li><li><p>The highest-energy electrons are in 3p, so we call sulfur a p-block element, and we don’t talk about its 3s orbital anymore.</p></li></ul></li></ul><p><span data-name="three" data-type="emoji">3⃣</span> In the d-block, the s orbital (ns) fills before the d orbital (n-1)d- (energy level before se.g. 3d), but both are still relevant.</p><ul><li><p>Example: Iron (Fe, Group 8, d-block)</p><ul><li><p>Electron configuration: [Ar] 4s² 3d⁶</p></li><li><p><strong>Even though 4s fills first, the highest-energy electrons are in 3d.</strong></p></li><li><p><strong>Since both 4s and 3d are involved, we include the s orbital when talking about d-block elements.</strong></p></li></ul></li></ul><div data-type="horizontalRule"><hr></div><p>Why Does the s Orbital Seem "Half-Filled" in d-Block Elements?</p><p>You might have noticed that some d-block elements only have one s electron instead of two (e.g., Cr → [Ar] 4s¹ 3d⁵).</p><p>This happens because:<br><span data-name="check_mark_button" data-type="emoji">✅</span><strong> A half-filled (d⁵) or fully filled (d¹⁰) d orbital is more stable than having a full s orbital.<br><span data-name="check_mark_button" data-type="emoji">✅</span> Electrons can "move" from the s orbital into the d orbital to create a more stable configuration.</strong></p><p>Example: <strong>Chromium (Cr, atomic number 24)</strong></p><ul><li><p><strong>Expected: [Ar] 4s² 3d⁴</strong></p></li><li><p><strong>Actual: [Ar] 4s¹ 3d⁵ (because a half-filled 3d⁵ is extra stable).</strong></p></li></ul><p><strong>But not all d-block elements do this! Many still have two s electrons, like iron (Fe) → [Ar] 4s² 3d⁶.</strong></p><div data-type="horizontalRule"><hr></div><p><strong>Final Takeaway (Short and Simple)</strong></p><p><strong><span data-name="check_mark_button" data-type="emoji">✅</span> p-block elements don’t “include” s orbitals because the p orbitals take over in the same energy level.<br><span data-name="check_mark_button" data-type="emoji">✅</span> d-block elements include s orbitals because the s orbital (ns) fills before the d orbital (n-1)d, and both remain relevant.<br><span data-name="check_mark_button" data-type="emoji">✅</span> Sometimes the s orbital only has one electron instead of two because the d orbitals become more stable when half-filled or fully filled.</strong></p><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/7b342a77-33f2-4dad-b114-959e650dc86c.jpg)
which orbital is the highest energy electron of group 1&2 metals found in?
s orbital
which orbital is the highest energy electron of group 3-0 atoms found in
p orbital
which orbital is the highest energy electron of transition metals found in
d orbital
maximum number of electrons in the s orbital
2
maximum number of electrons in the p orbital
6
maximum number of electrons in the d orbital
10
maximum number of electrons in the f orbital
14
maximum number of electrons in principle quantum 1?
2
maximum number of electrons in principle quantum 2?
8
maximum number of electrons in principle quantum 3?
18
what is the order of orbitals in increasing energy?
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p
why do electrons spin in opposite directions?
to minimise repulsion
what is ionisation?
when electrons have been removed from an atom or molecule
ionisation definition and 2nd ionisation enrgy defintion
the amount of energy required to move 1 mole of electrons from 1 mole of atoms in gaseous state
the amount of energy required to move 1 mole of electrons from 1 mole of ions in gaseous state
what is the first ionisation energy?
the energy needed to remove 1 electron from each atom in 1 mole of gaseous atoms to form 1 mole of gaseous 1+ ions
what is successive ionisation?
the removal of more than 1 electron from the same atom is called successive ionisation
what state symbol do we use for ionisation energy?
g
what does it mean if an atom has a high ionisation energy?
- high attraction between the electron & the nucleus
- more energy is needed to remove the electron
what factors affect ionisation energy?
- nuclear charge
- distance from nucleus
- shielding
how does nuclear charge affect ionisation energy?
- more protons in the nucleus = nucleus is more positively charged so stronger attraction for the electrons
how does distnce from nucleus affect ionisation energy?
the further the electron is from the nucleus, the weaker the attraction
how does shielding affect ionisation energy?
as shielding increases, attraction between outer electrons and nucleus decreases
why is the 2nd ionisation energy higher than the 1st ionisation energy?
- the electron is being removed from a positive ion
- requires more energy
what is the general equation for the nth ionisation?
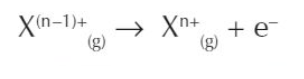
what is the ionisation trend down groups e.g. group 2?
first ionisation energy decreases down group 2
increased shielding- more shells between nucleus and outer shell so attractive force is weaker
atomic radius increases, outer electrons are further away from the nucleus
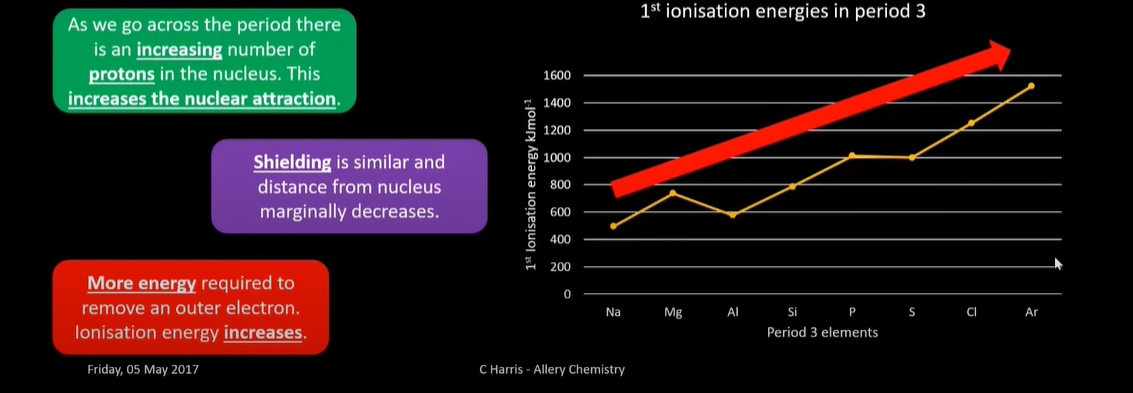
what is the general ionisation trend across a period? And exceptions?
increases
exceptions:
a decrease at alluminum is evidence for atoms having sub shells
the outermost electron in Aluminium sits in a higher energy subshell slightly further from the nucleus than the outer electron magnesium. The atomic model Niels Bohr came up with didn’t exlain subshells
a decrease at sulfur is evidence for electron repulsion in an orbital
removing an electron from sulfur involves taking it from an orbital with 2 electrons in
electrons repel eachother so less energy is needed to remove an electron from an orbital with 2 in it than 1
g to mg
x1000
Define the term relative atomic mass. (2)
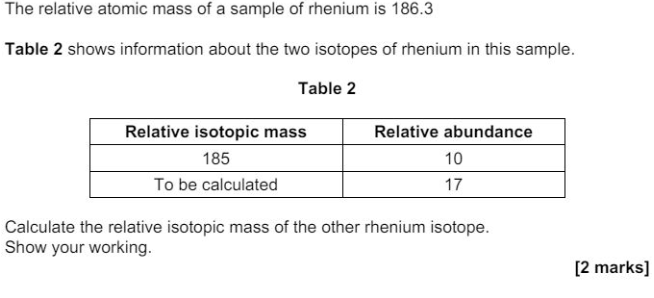
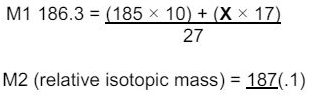
"State how the relative abundance of 185Re+ is determined in a TOF mass spectrometer. (2)
at the detector/(negative) plate the ions/Re+ gain an electron (relative) abundance depends on the size of the current

