VIRO 2 | Entry (Adsorption, Penetration, Unocoating)
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
General steps to virus replication cycle
apunmr
Adsorption
Penetration
Uncoating
Maturation (assembly)
Release
_ refers to the process when virus particles attach to molecules on host cell surface
Adsorption
Host cell receptors can be _, serving various cellular functions, e.g., receptors, adhesion molecules, transporters rat
pcl
Proteins
Carbohydrates
Lipids
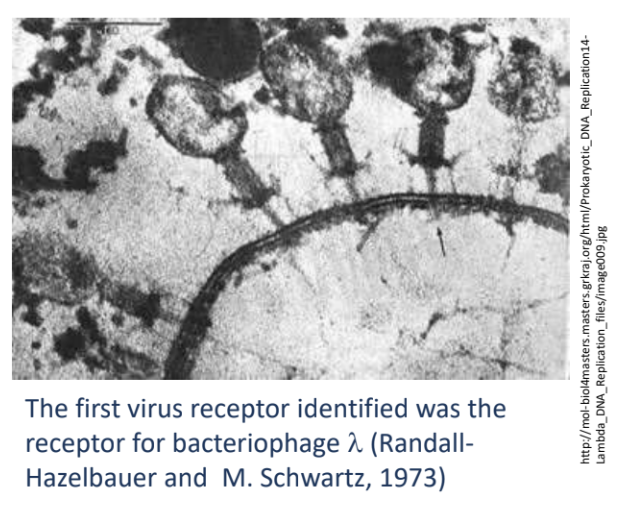
The first virus receptor identified was the receptor of _
bacteriophage lambda (responsible for maltose uptake)
T/F: Some viruses do not require adsorption to infect
TRUE
Some viruses enter through cell damage and thus do not need to adsorb to host cell surface molecules
T/F: Virions can simply diffuse across host lipid bilayer
FALSE
Virions are too big and cannot make use of amphiphilic nature of lipid bilayer, protein channels, nor enter directly (unless they enter via cell damage)
T/F: There is usually specific virion-host receptor interaction
TRUE
T/F: Even if the host doesn’t have the receptor for a specific virus, the virus can still enter the cell, except for fungal/yeast and plant viruses
FALSE
Viruses generally cannot enter host cell if host cell doesn’t have receptor specific to virus
Host cell receptors can be proteins, carbohydrates, lipids, serving various cellular functions, including _
rat
Receptors
Adhesion molecules
Transporters
There is usually a specific virion-host receptor interaction, except for _
Fungal and yeast viruses bc they don’t have an extracellular phase and are just vertically/horizontally passed on from one fungal cell to another
Plant viruses bc they enter via cell wall damage by arthropod vectors
T/F: All animal viruses require host cell receptors
TRUE
_ is one of the earliest identified receptor for animal virus, e.g., influenza
Sialic acid
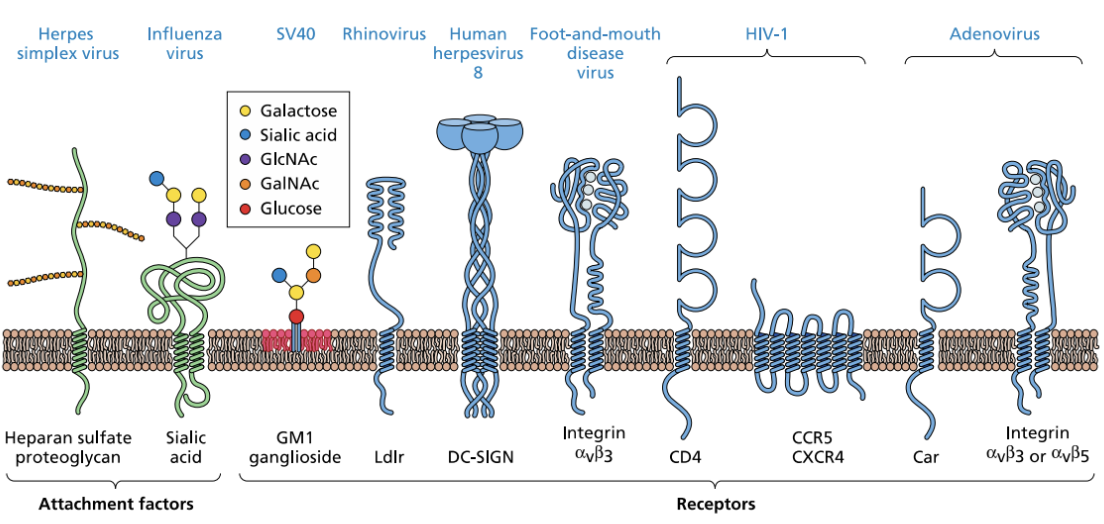
Enumerate and explain some examples of attachment factors and receptors
Attachment factors
Heparan sulfate proteoglycan
Sialic acid = glycoprotein
Receptors
HIV = CCR5, CXCR4
Adenovirus = Car, Integrin
T/F: It is possible to have two receptors, one of which is a co-receptor required for attachment and entry of virus
TRUE
e.g., HIV
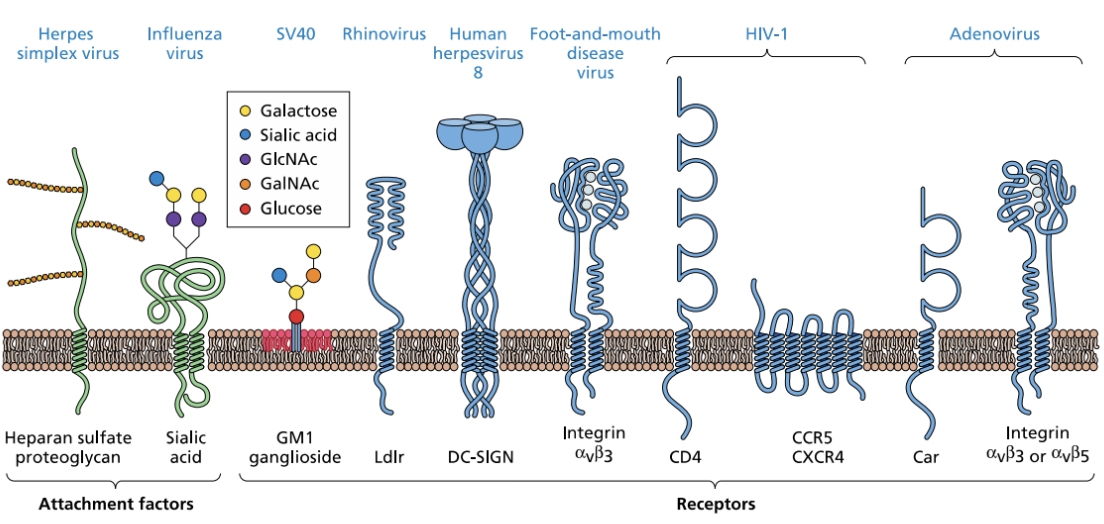
T/F: Galactose, sialic acid, GlcNAc, GalNAc, and glucose are some common components of host cell receptors
TRUE
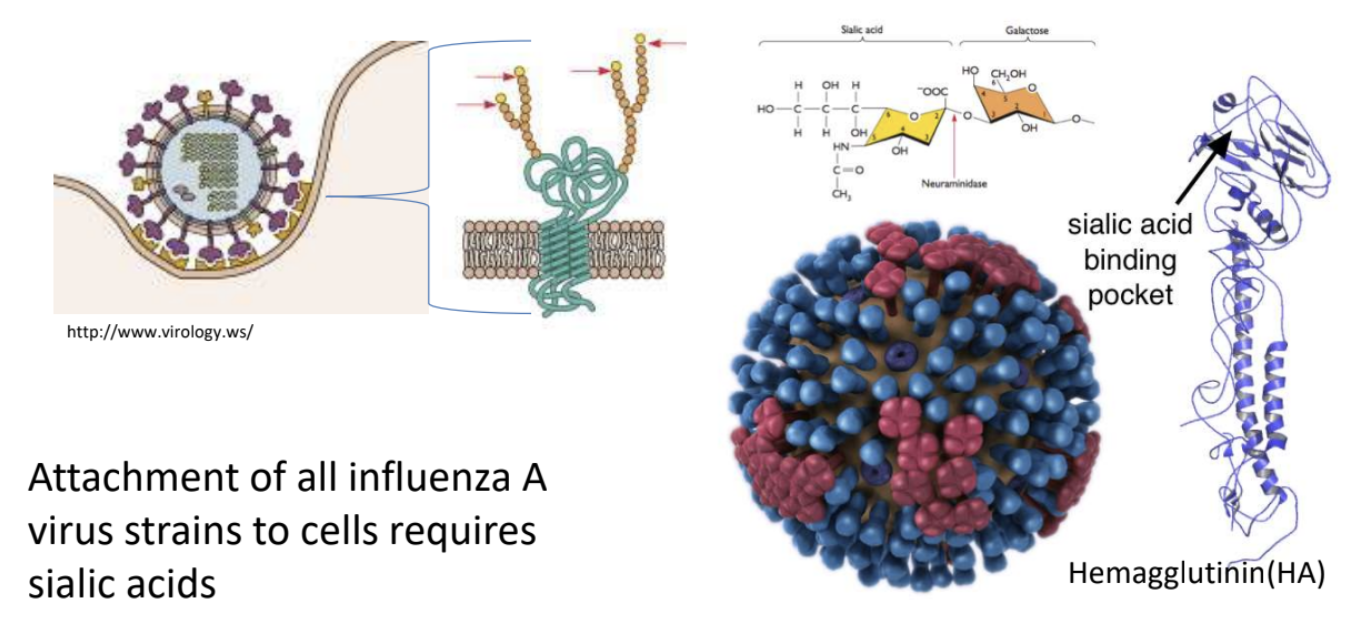
The attachment of all influenza A virus strains to cells requires _ as receptors
sialic acids *terminal sugar attached to oligosaccharide on glycoprotein
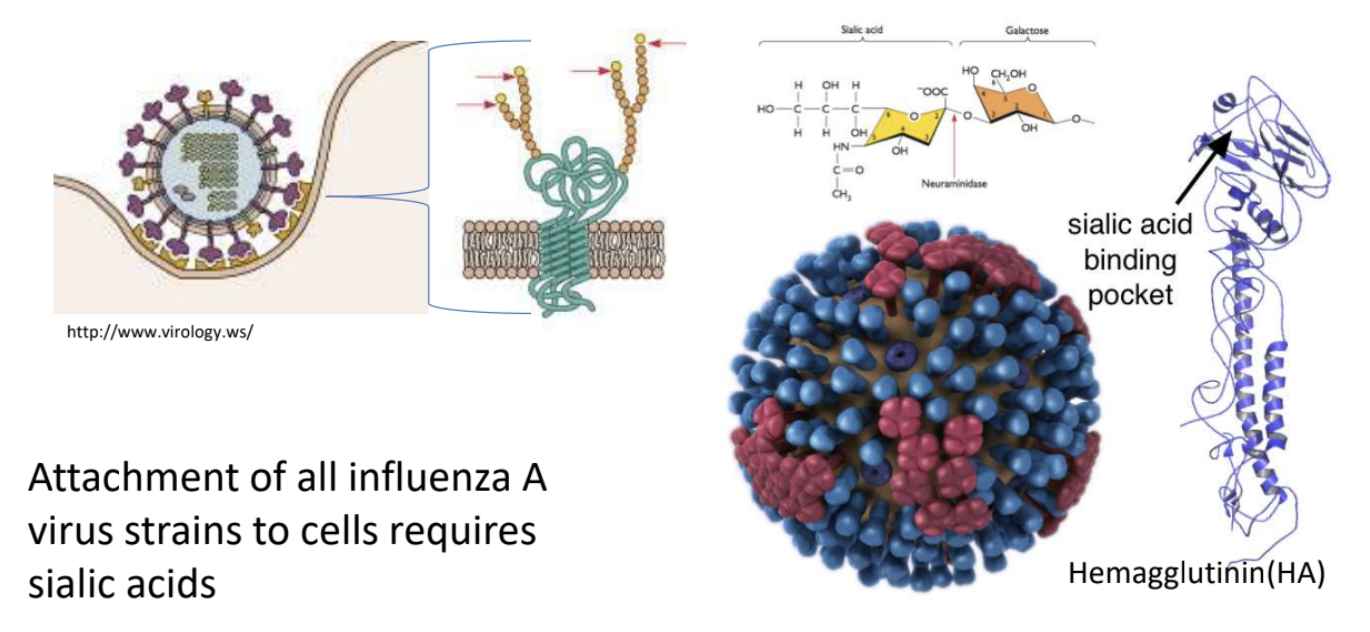
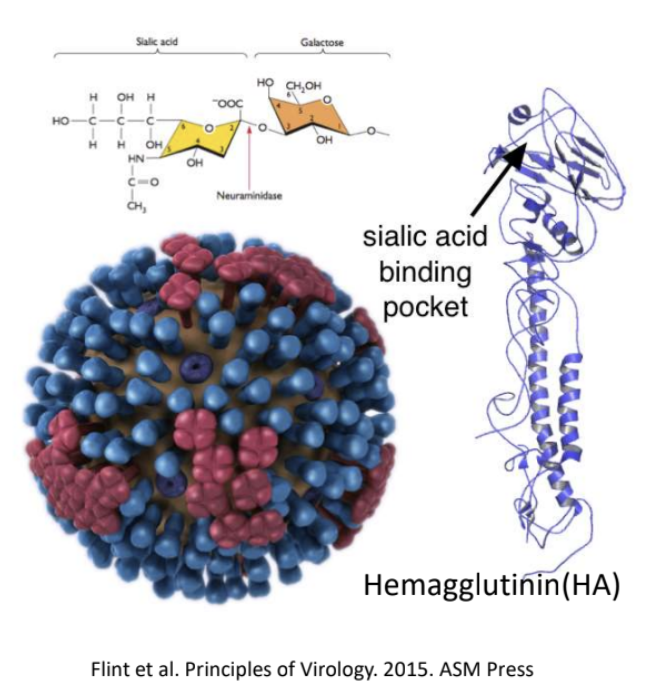
_ is the one that recognizes sialic acid on host cell surface
Hemagglutinin (HA)
Explain the different sialic acids recognized by influenza viruses
Avian influenza virus = a(2,3) linked sialic acids in duck gut
Human influenza virus = a(2,6) linked SA in human respiratory epithelial cells, but a(2,3) linked is also found in ciliated epithelial cells in a minor population in human resp tract
Porcine = both a(2,3) & a(2,6) in trachea; hence, a melting pot for emergence of new viruses
T/F: Humans can be directly infected by avian influenza virus
TRUE
Bc we have a(2,3) linked SAs recognized by avian influenza viruses, but it’s in ciliated epithelial cells in our respiratory tract, thus the chances are low bc most likely, this will have been combatted already by our immune system before it gets to respiratory tract
T/F: The attachment of all influenza A virus strains to cells require heparan sulfate proteoglycan as receptors
FALSE
The attachment of all influenza A virus strains to cells requires sialic acids as receptors
What makes pigs a melting pot for emergence of new viral diseases?
Pigs express both a(2,3) and a(2,6) linked SAs, thus allowing them to have receptors that can be recognized by avian and human influenza and subsequently making them a “mixing vessel” for influenza viruses, as this can facilitate reassortment and the potential emergence of new pandemic strains
How is a farm a very important surveillance point for influenza viruses?
This is where the interaction between 3 species with different sialic acid receptors happens, potentially facilitating reassortment and resulting in the emergence of new viral diseases
T/F: Highly pathogenic avian influenza (HPAI) viruses were found to directly infect cows and humans
FALSE
Bird > cow, cow > humans
Humans were not directly infected
T/F: There is evidence for avian influenza virus spreading from humans to humans
FALSE
Sequence of SARS-CoV-2, including its receptor-binding motif (RBM) that directly contacts _, is similar to SARS-CoV, suggesting that SARS-CoV-2 uses _ as its receptor
ACE2
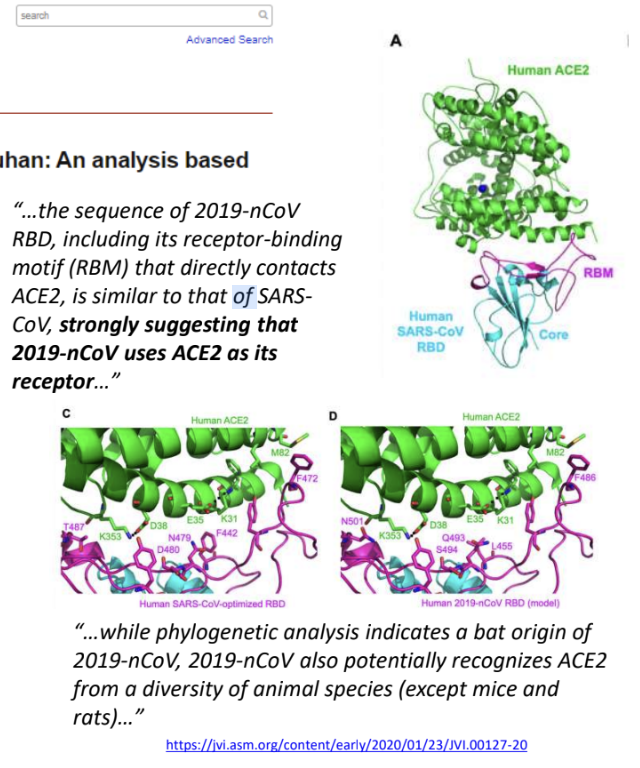
While phylogenetic analysis indicates a bat origin of SARS-CoV-2, SARS-CoV-2 potentially recognizes _
ACE2 from various animal species, except from mice and rats (whose ACE2 receptors do not effectively bind SARS-CoV-2)
T/F: No human to human spread of avian influenza virus (H5N1) has ever been detected in dairyhouse in US in 2019
TRUE
Bc there are very low chances for bird > human transmission
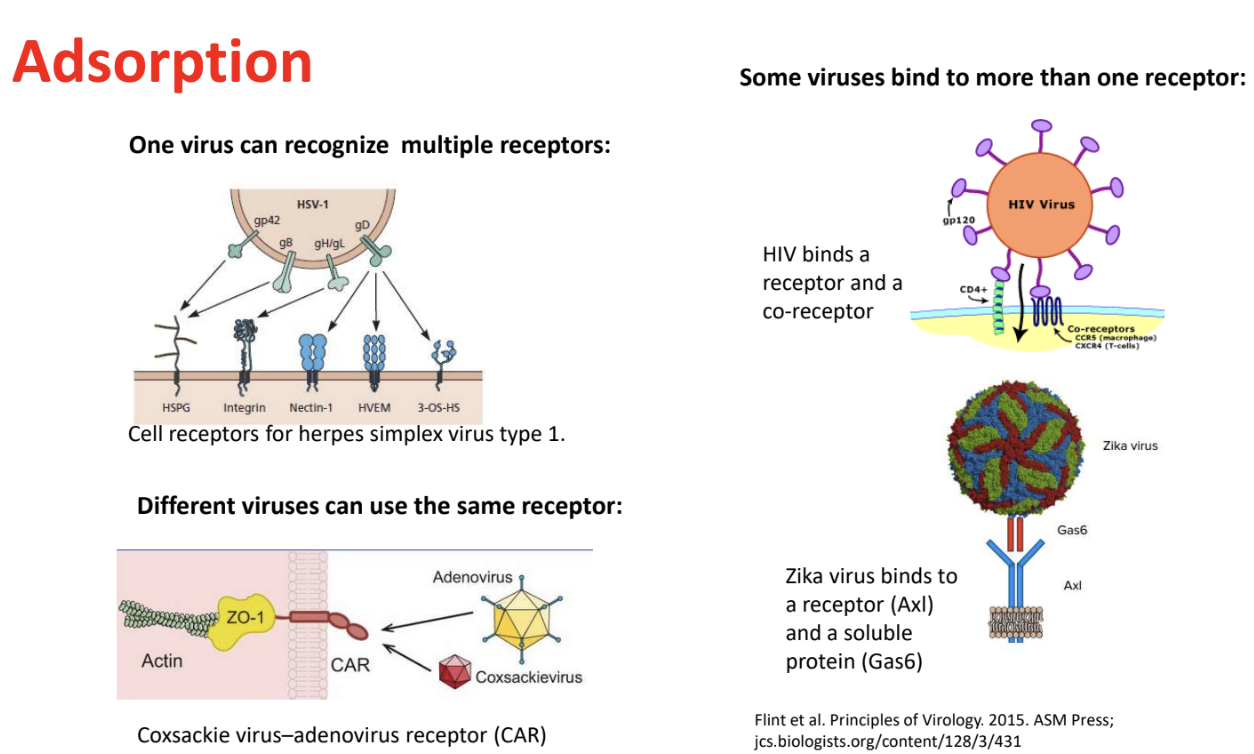
Explain the different interactions existing between viruses/virus surface proteins and their host receptors
Virus (surface proteins) can recognize multiple host receptors
V1 → R1, R2, or R3
e.g., Herpes simplex virus type 1 (HSV-1)
Different viruses can use the same receptor
V1, V2 → R1
e.g., Coxsackie & Adenovirus → Car (Coxsackie-Adenovirus receptor)
Some viruses bind to more than 1 receptor (receptor + co-receptor)
V1 → R1 & R2
e.g., HIV → CD4+ & CCR5 (macrophage) CXCR4 (T-cells)
receptor + co-receptor
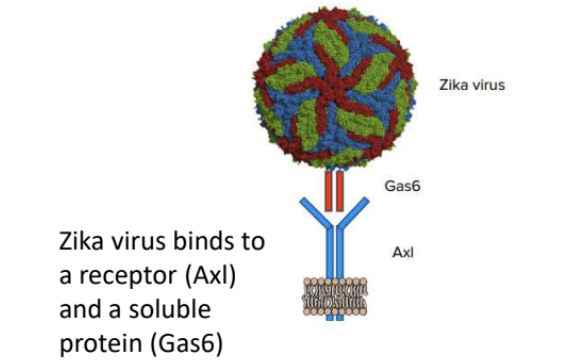
e.g., Zika virus → Axl & Gas
soluble protein + receptor
Zika virus is unusual because for it to bind to a host cellular receptor (Axl), it must first _
bind to a soluble protein (Gas6)
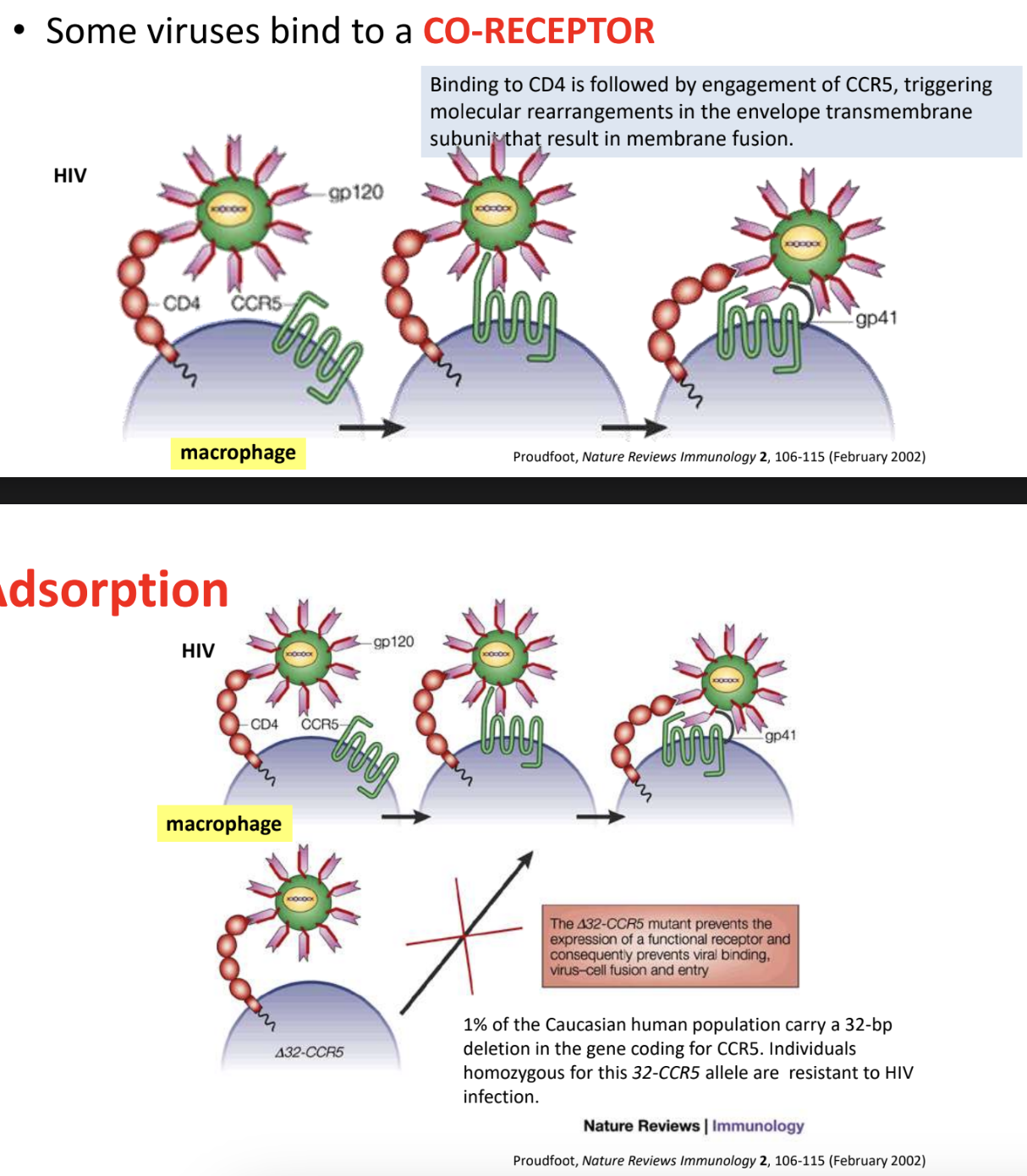
Explain adsorption receptor + co-receptor binding principle/process in HIV
gp120 protein in HIV viral envelope specifically interacts with CD4 host cell receptor, causing a conformational change in gp120 that exposes a hidden binding site for a co-receptor
Conformational change in gp120 leads gp120 to interact initially with CCR5 (macrophage-tropic), later on switching to CXCR4 (T-cell-tropic)
Binding of gp120 with co-receptors then triggers gp41 to insert its fusion peptide into host cell membrane, facilitating fusion of viral envelope + host cell membrane
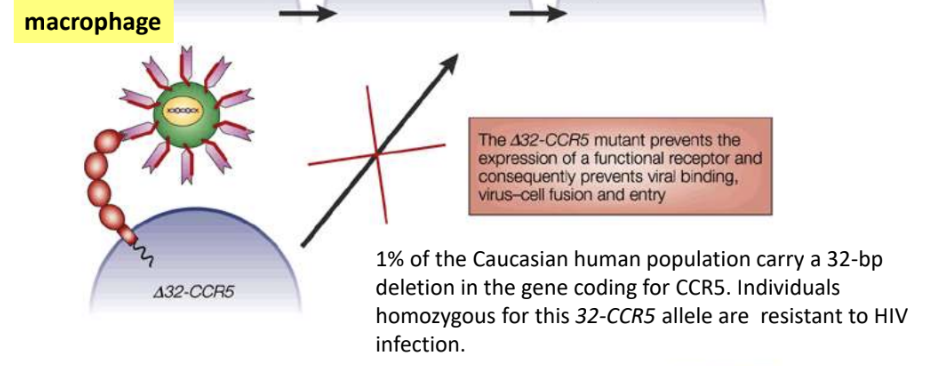
*1% of Caucasian human population carry 32-bp deletion in gene coding for CCR5, thus individuals homozygous for this deletion (32-CCR5 allele) = resistant to HIV infection
Explain the change in cellular tropism in HIV as disease progresses
gp120 first binds to CD4 host receptor, triggering conformational change that allows co-receptor binding
Initially (early in the infection), gp120 binds to CCR5 receptor in macrophages, thus B-chemokines can inhibit this binding
Later on, gp120 binds to CXCR4 receptors in T-cells, causing severe immunodeficiency; a-chemokines inhibit this binding
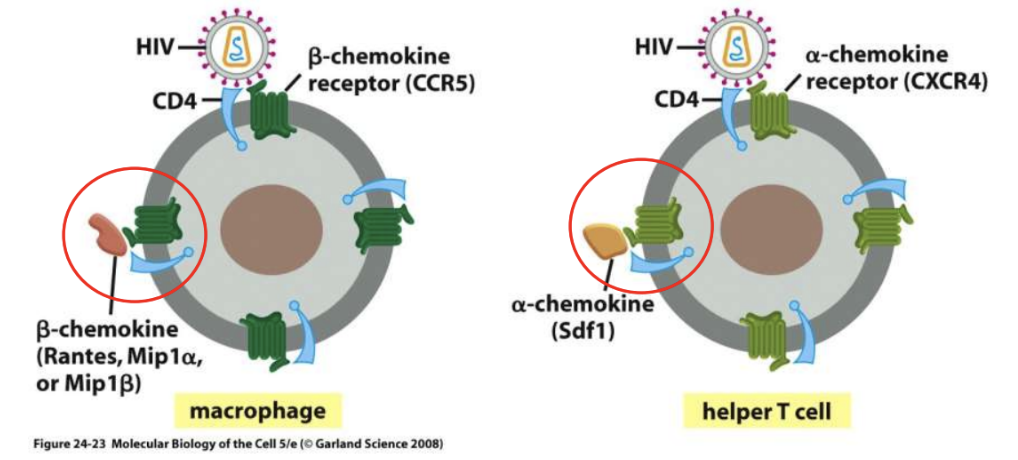
_ inhibits binding of gp120 in HIV to CCR5 (macrophages)
B-chemokines
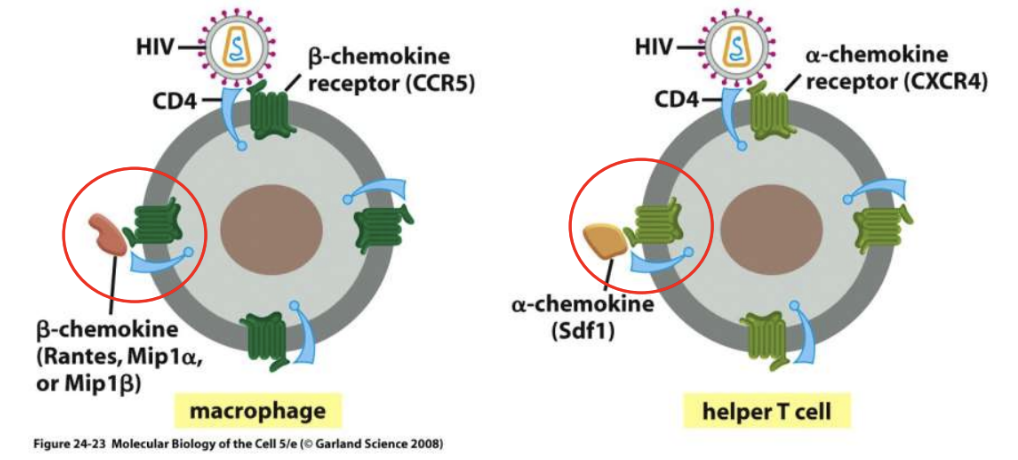
_ inhibits binding of gp120 in HIV to CXCR4 (T-cells)
a-chemokines
Sequence of SARS-CoV-2, including its _ that directly contacts ACE2, is similar to SARS-CoV, suggesting that SARS-CoV-2 uses ACE2 as its receptor
receptor-binding motif (RBM)
_ that block the interaction of HIV-1 envelope with CCR5 or CXCR4 potently inhibit HIV-1 in vitro
Pilot studies of _ have provided proof of concept for this novel drug class; phase III safety and efficacy trials are underway
Chemokine receptor antagonists
orally bioavailable small molecule CCR5 inhibitors

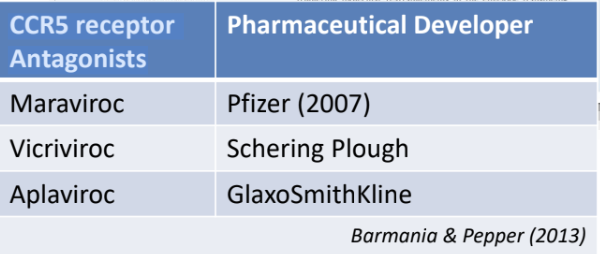
Enumerate three CCR5 receptor antagonists currently being developed
mva
Maraviroc = Pfizer (2007)
Vicriviroc = Schering Plough
Aplaviroc = GlaxoSmithKline
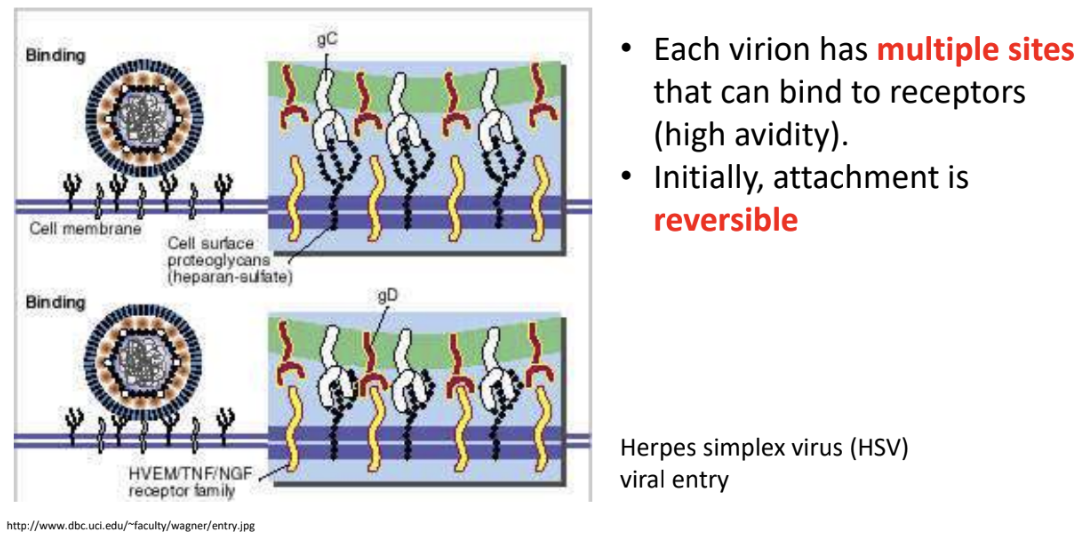
What stabilizes virus-receptor interaction?
Each virion has multiple sites that can bind to receptors (high avidity)
Initially, attachment is reversible since the bond is noncovalent and there will only be a few attachment/interactions, but over time, as this increases, the virus-receptor interaction becomes more stable and eventually irreversible
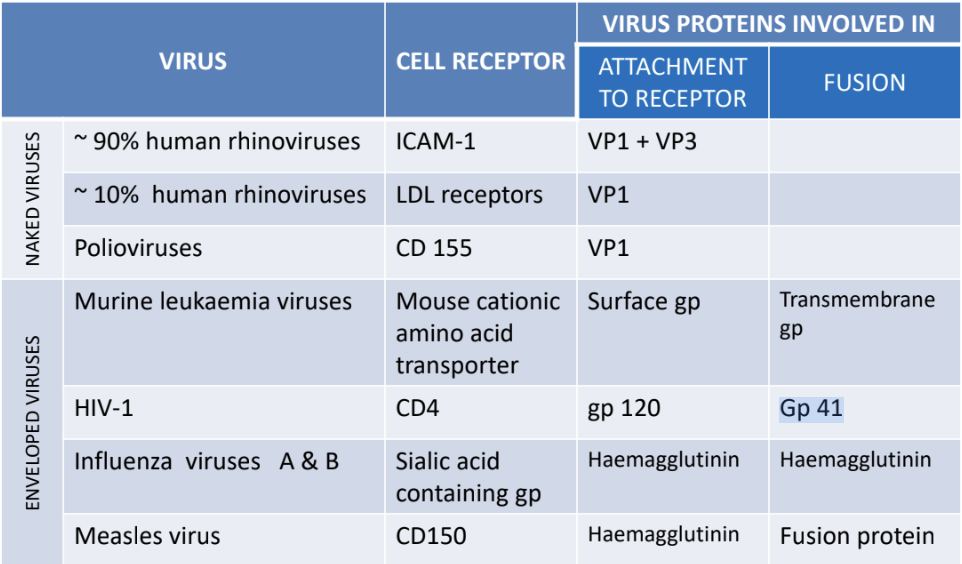
Explain adsorption (n & e) viruses + virus proteins for attachment & fusion + host receptors
Viruses | Virus proteins (for attachment; fusion) | Host cell receptors |
N: ~90% human rhinoviruses | VP1+VP3 | ICAM-1 |
N: ~10% human rhinoviruses | VP1 | LDL receptors |
N: Polioviruses | VP1 | CD 155 |
E: Murine leukemia | surface gp; transmembrane gp | Mouse cationic AA transporter |
E: HIV-1 | gp120; gp41 | CD4 |
E: Influenza A & B | Hemagglutinin (HA); HA | Sialic acids with gp |
E: Measles | HA; fusion protein | CD 150 |
1% of Caucasian human population carry _, thus individuals homozygous for this deletion are resistant to HIV infection
32-bp deletion in gene coding for CCR5
Explain how to gather experimental evidence to determine if a cell surface molecule is a virus (host cell) receptor
nmse
If the normal ligand for cell surface molecule blocks virus binding / infectivity, e.g., excess CD4 ligands prevent HIV from binding to CD4, then this implies that HIV competes with CD4 ligands and CD4 is a receptor
If the monoclonal antibody against cell surface molecule blocks virus binding/infectivity, e.g., anti-CD4 antibodies prevent HIV from binding to T-cells, then this implies that CD4 is required for HIV entry
If a soluble derivative of cell surface molecules blocks virus binding / infectivity, e.g., soluble CD4 proteins bind HIV and prevent its infection; CD4 is a receptor
If the expression of gene encoding cell surface molecule in a virus-resistant cell causes that cell to be susceptible to infection, then this suggests that CD4 is required for HIV entry
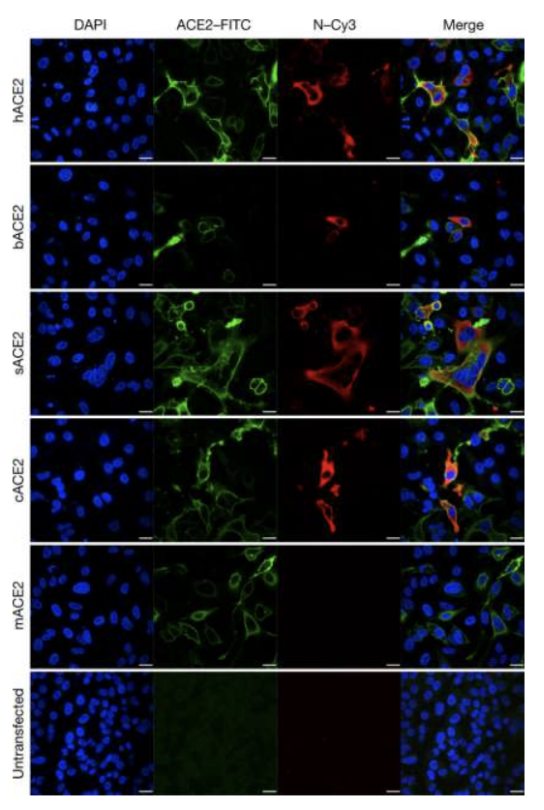
_ is an inherent barrier to infection, e.g., mice and rats cannot get infected with SARS-CoV-2 because their ACE2 receptors do not effectively bind the said virus
Lack of membrane receptors

T/F: Murine models can be used for studying mechanisms of SARS-CoV-2
FALSE
This cannot be used because mice are resistant to SARS-CoV-2 because their ACE2 receptor do not effectively bind the said virus
_ is sufficient to make the cells susceptible to infection
Providing the receptor protein
Explain acquisition of phage sensitivity by bacteria through exchange of receptors (adsorption > membrane receptors = susceptible to infection)
Phage-resistant (R) bacteria become lysed when co-cultured with sensitive (S) bacteria
R cell lysis is triggered by phage lysins released from nearby infected S cells
Occasionally, phages invade R cells only when co-incubated with S cells and this is because invasion is mediated by R cells gaining attachment molecules from S cells (most likely via HGT)
*Gain of attachment molecules allow entry of phages that cause lysis
*B. subtilis, lytic phage SPP1
_ refers to the phenomenon of resistant cells infected by phages in mixed cultures
acquisition of sensitivity (ASEN)
_ is when purified phage lysis proteins lyse cells from the outside, even in an interspecies manner
“Lysis from without”
T/F: The presence of phage-sensitive population within bacterial community could dramatically impact population dynamics via LO and ASEN
TRUE
Explain some therapeutic strategies conceptualized for 2019-nCoV
Introduce:
Soluble components of RBD of SARS-CoV-2 to block its binding to receptor
Antibody fragments specific to receptor to block virus binding
Antibody fragments which bind to virus, blocking binding of virus to receptor
Ligands binding to receptor and thus preventing virus from binding to receptor
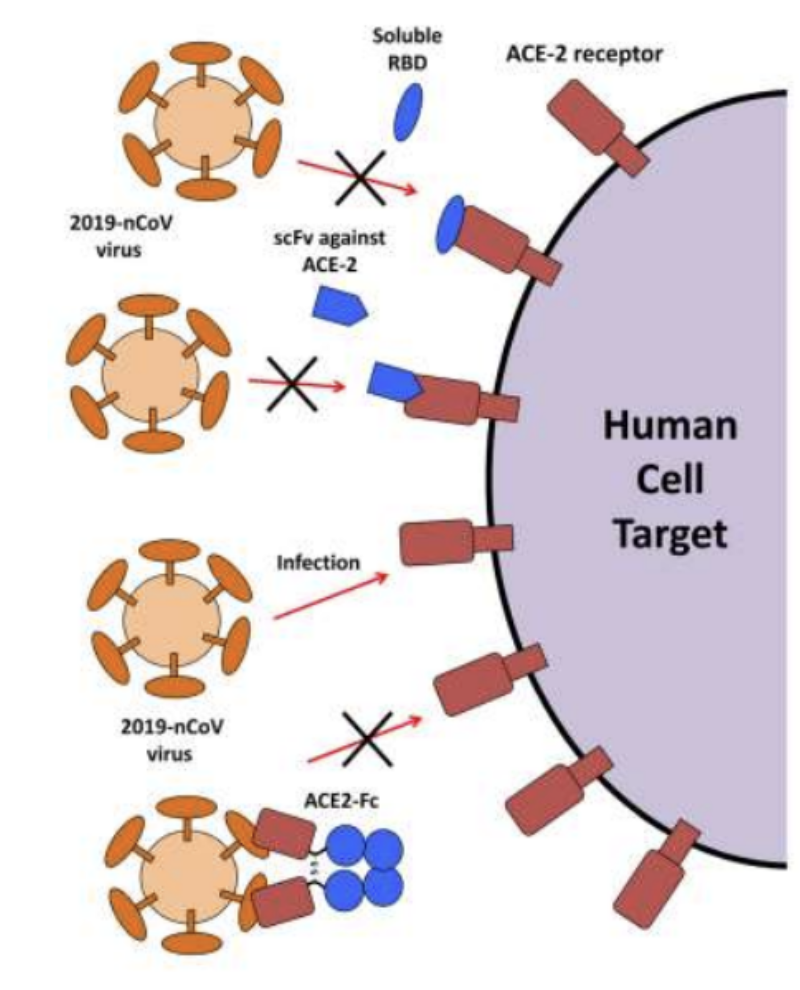
_ are specifically designed to target the receptor binding domain (RBD) of SARS-CoV-2, preventing the virus from binding to the receptor and thus neutralizing it
Monoclonal antibodies
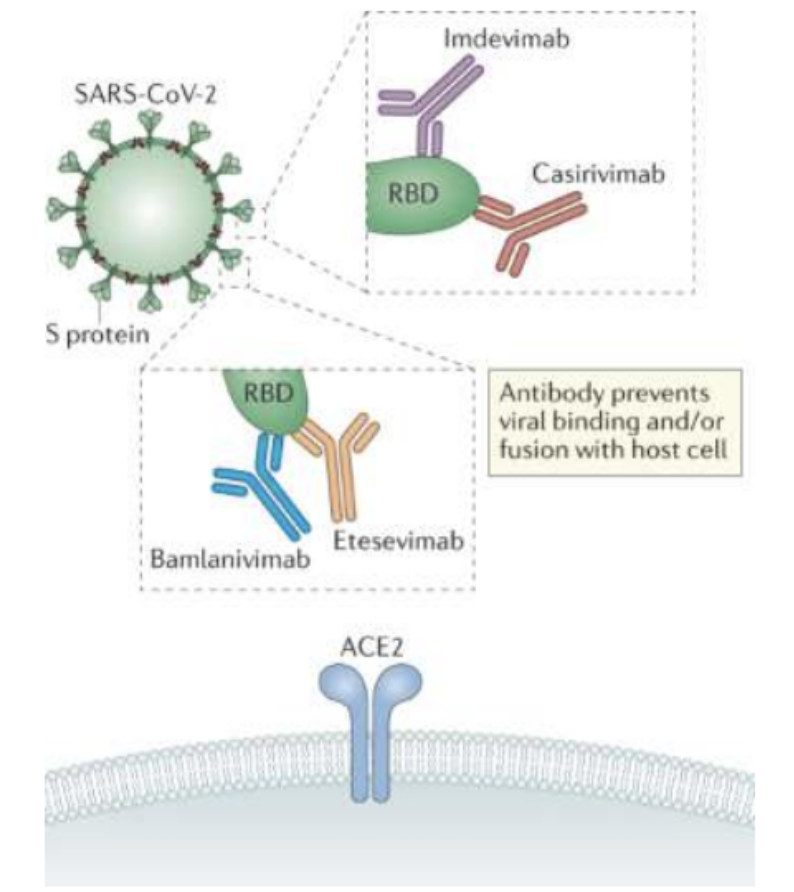
T/F: There is constant host-virus arms race
TRUE

How come mutations that prevent viral interaction with host haven’t predominated in the population?
It’s because there is a limitation to the amount of mutations that virus receptors can undergo, given that these still have normal functions within the host body and thus cannot constantly undergo mutation
Hosts typically have slower mutation rate compared to viruses
Red queen hypothesis = co-evolution
T/F: Naked viruses typically have different proteins for attachment to receptors and fusion with host cell membrane
FALSE
Enveloped viruses typically have different proteins for attachment to receptors and fusion with the host cell membrane
_ refers to the human-specific receptor variant that confers partial protection from severest rhinovirus (RV-C)-induced asthma episodes
CDH3-Cys529
Influenza is an enveloped virus undergoing penetration and uncoating via _
entire virus taken up via receptor-mediated endocytosis > fusion & uncoating
_ is a disease that causes wheezing illness and asthma exacerbations
Rhinovirus (RV-C)
HIV is an enveloped virus undergoing penetration and uncoating via _
fusion of viral envelope + host cell membrane > uncoating of viral genome
All animals are known to maintain a receptor gene (for RV-C) with high level of sequence conservation known as _
CDH3-Tyr529

T/F: CHD3-Tyr529 increases risk of wheezing illness compared to human WT CDH3-Ser529
FALSE
CHD3-Tyr529 increases risk of wheezing illness compared to human WT CDH3-Cys529

_ refers to the entry of viruses into cells and removal of capsid
Penetration and uncoating
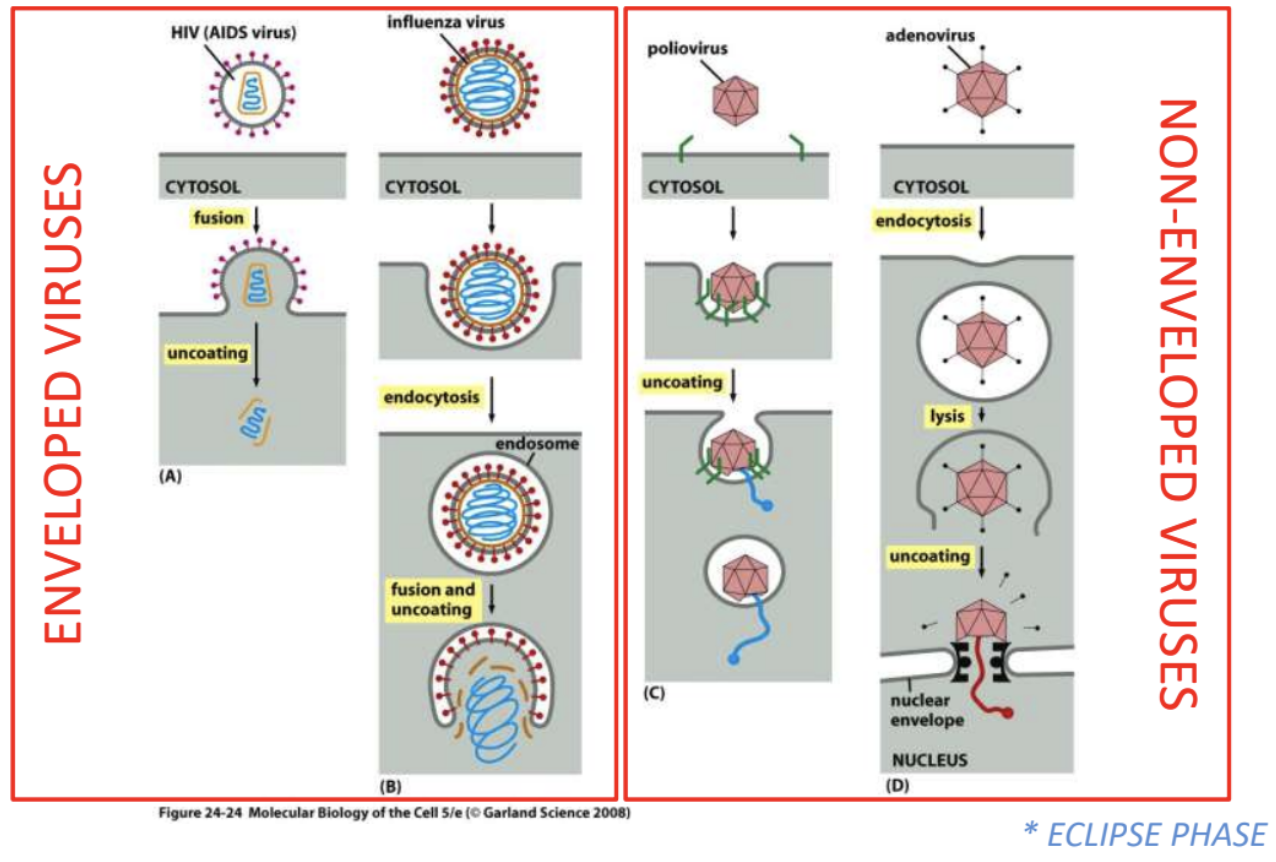
Explain penetration and uncoating strategies of enveloped viruses vs. naked viruses
Enveloped
HIV = fusion > uncoating
Fusion of viral envelope with host cell membrane, leading to subsequent uncoating of viral genome, so that it’s transported to host cell
Influenza = endocytosis > fusion & uncoating
Entire virus is taken up via receptor-mediated endocytosis, then pH levels in endosome trigger fusion of viral envelope + host cell membrane, leading to uncoating of viral genome
Naked
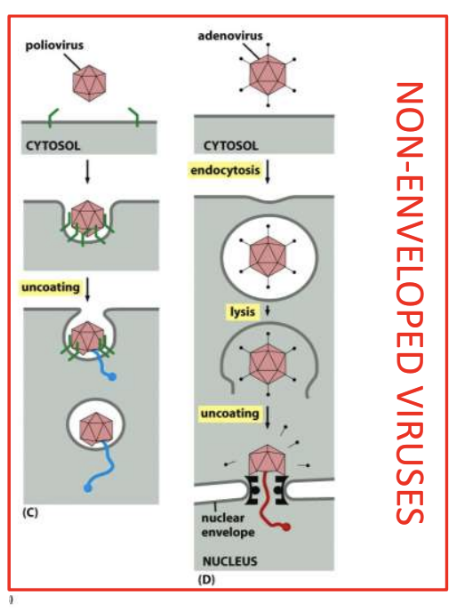
Poliovirus = partial degrading of capsid > extrusion of NA
Capsid is partially broken down, then NA is extruded into host cell
Adenovirus = endocytosis > partial degrading of virion > uncoating & delivery to nucleus
Naked virus is taken up via receptor-mediated endocytosis
Inside cell, partially degraded virion is directed to nuclear envelope where genome is uncoated and delivered to nucleus
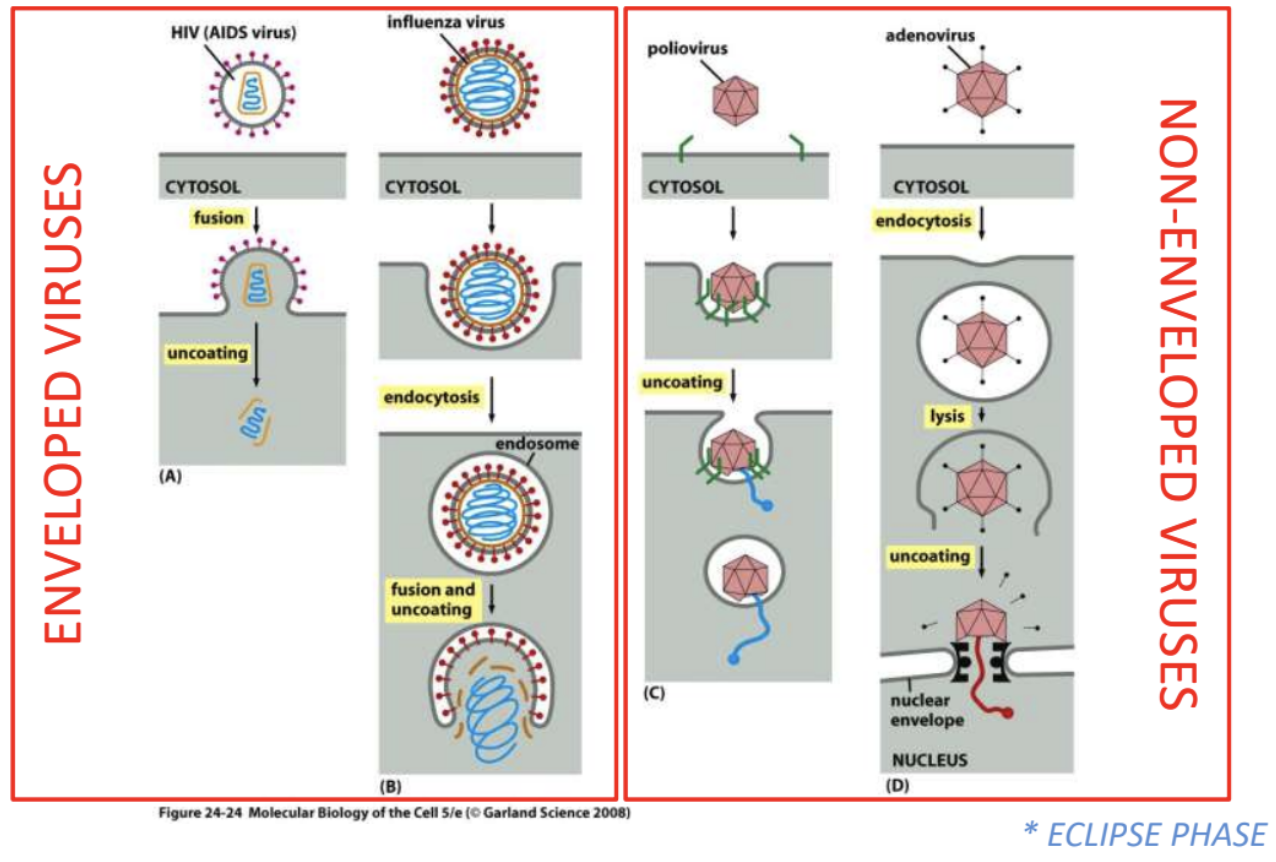
_ refers to the phase when virion is uncoated and nucleocapsid is released into host cell, thus no intact virions are detected inside the cell, lasting for as long as there is still no new assembled viral particles in the host cells
Eclipse phase
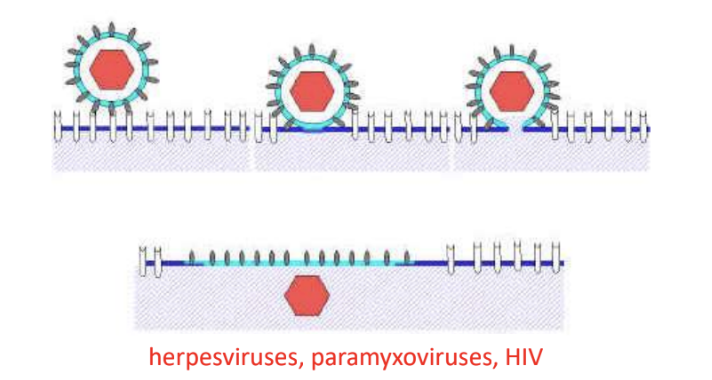
Give 3 examples of viruses that penetrate host cell via direct fusion of viral envelope with host plasma membrane
phh
Paramyxoviruses
Herpesviruses
HIV
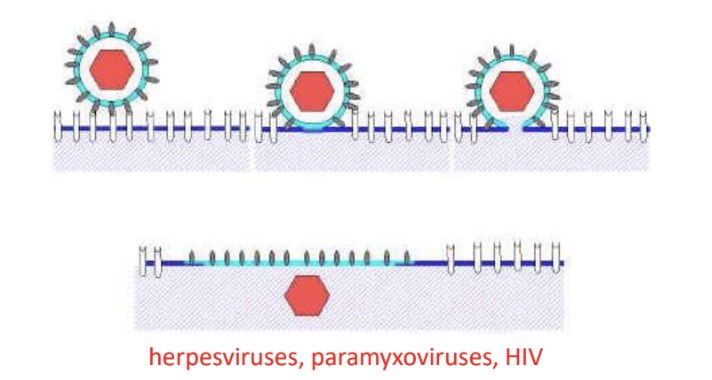
Elaborate on virus penetration via direct fusion of viral envelope with host plasma membrane (in enveloped viruses)
afn
Occurs after attachment to host cell receptor
Fusion is promoted by fusion protein
Nucleocapsid is directly released into cytoplasm
e.g., Paramyxo, herpes, HIV
_ facilitates the fusion of viral envelope with host plasma membrane
Fusion proteins
e.g., transmembrane proteins in murine leukemia,
gp41 in HIV
HA in influenza A & B
fusion protein in measles
T/F: Fusion sequence of fusion proteins is mostly made up of hydrophobic sequences
TRUE
So it can insert itself into target membrane
Why is fusion protein normally hidden away?
*Normally (in HIV), fusion protein is only accessed after binding of attachment viral protein (gp120) to co-receptor (CCR5, CXCR4)
For the fusion process to be regulated because if you just have a series of hydrophobic AA sequences exposed, then virion can just fuse to any nearby lipid bilayer
T/F: Surface subunit (SU) is gp120 and transmembrane subunit (TM) is gp41 in HIV; both are viral proteins but only gp120 interact to cell receptors
TRUE
Explain how fusion occurs in an HIV-1
Binding of HN protein to its cellular receptor induces conformational changes, which triggers also a conformational change in fusion protein, exposing fusion peptide and making protein fusion-competent
Binding of gp120 (SU) to CD4 exposes a high-affinity chemokine receptor-binding site on SU
SU-CCR interaction leads to conformational changes in gp41 (TM) that exposes fusion peptide and permits it to insert into cell membrane, catalyzing fusion
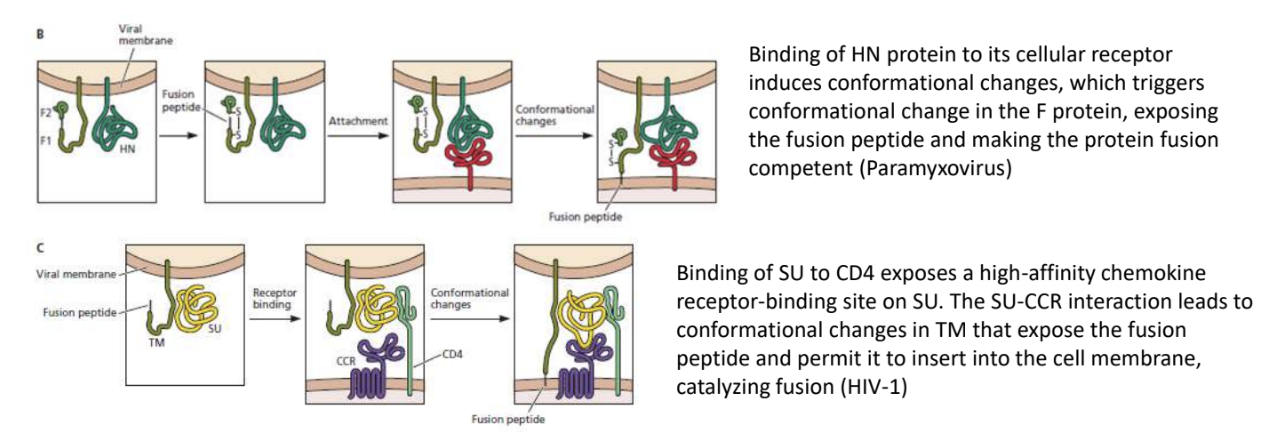
In HIV-1, the conformational change that exposes fusion sequence is triggered by _
virus binding to a receptor
_ leads to conformational change in TM (gp41) that expose fusion peptide, allowing it to be inserted into cell membrane and catalyzing fusion
SU(gp120)-CCR interaction
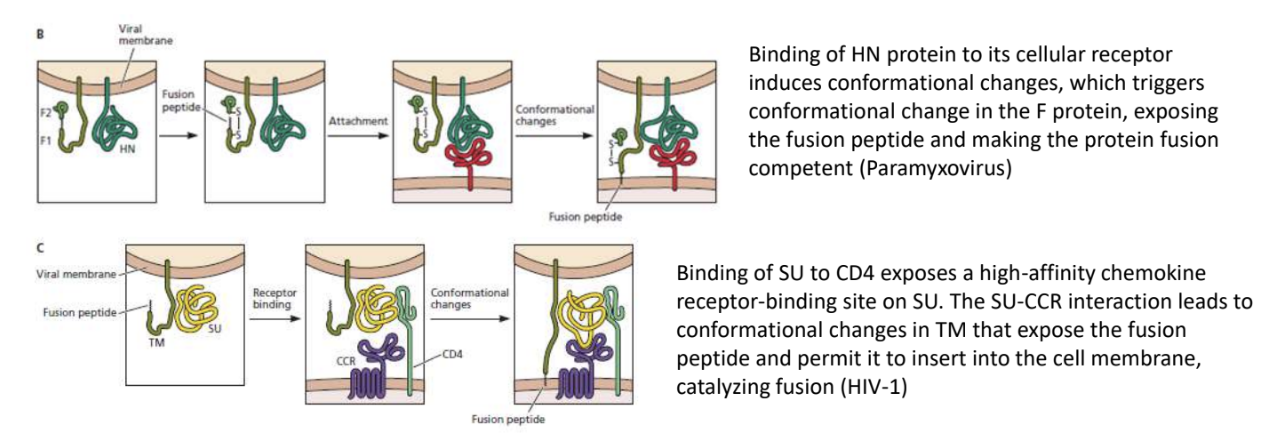
In HIV-1, _ exposes a high-affinity CCR5-binding site on gp120
_ interaction leads to conformational change in gp41 that exposes fusion peptide and permits it to be inserted into cell membrane, catalyzing fusion
Binding of gp120 (viral attachment protein) to CD4 (host cell receptor)
gp120-CCR5
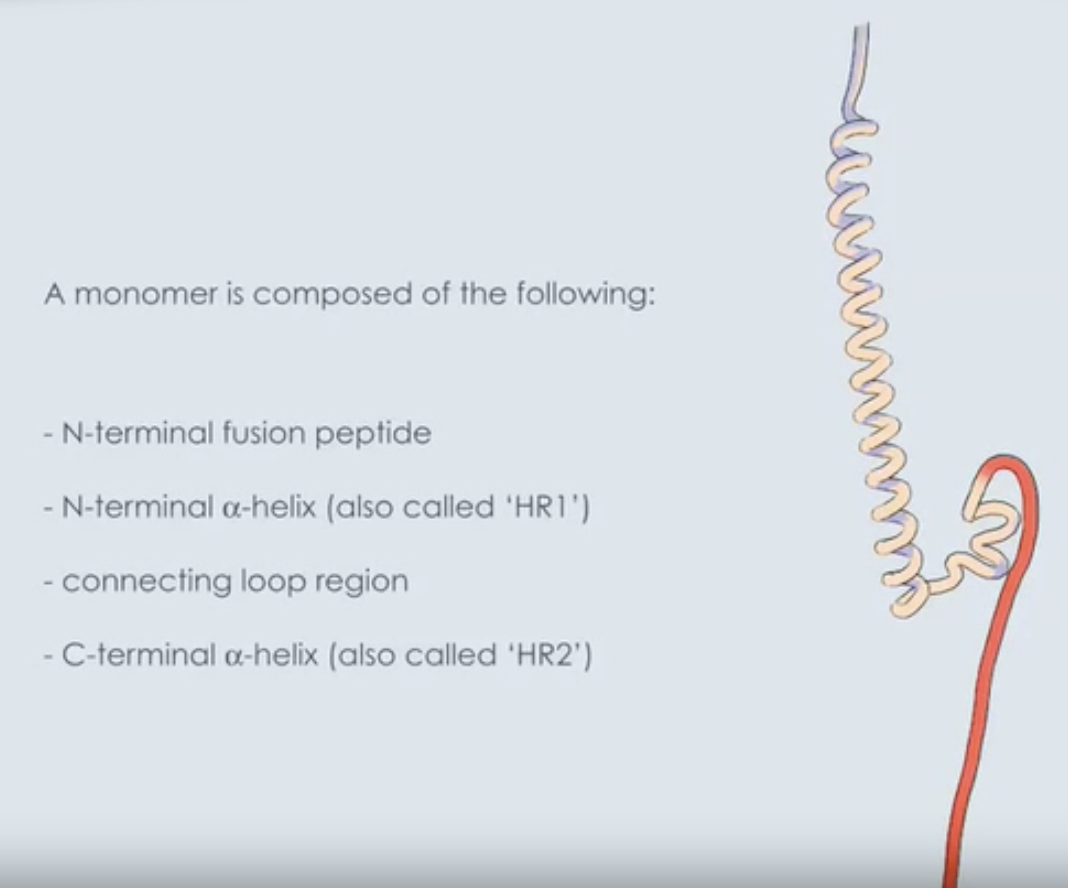
T/F: During the fusion process in HIV-1, the C-terminal region (HR2) anchored in viral membrane undergoes B-helical transition and runs alongside outside of core
FALSE
During the fusion process in HIV-1, the C-terminal region anchored in viral membrane undergoes a-helical transition and runs alongside outside of core
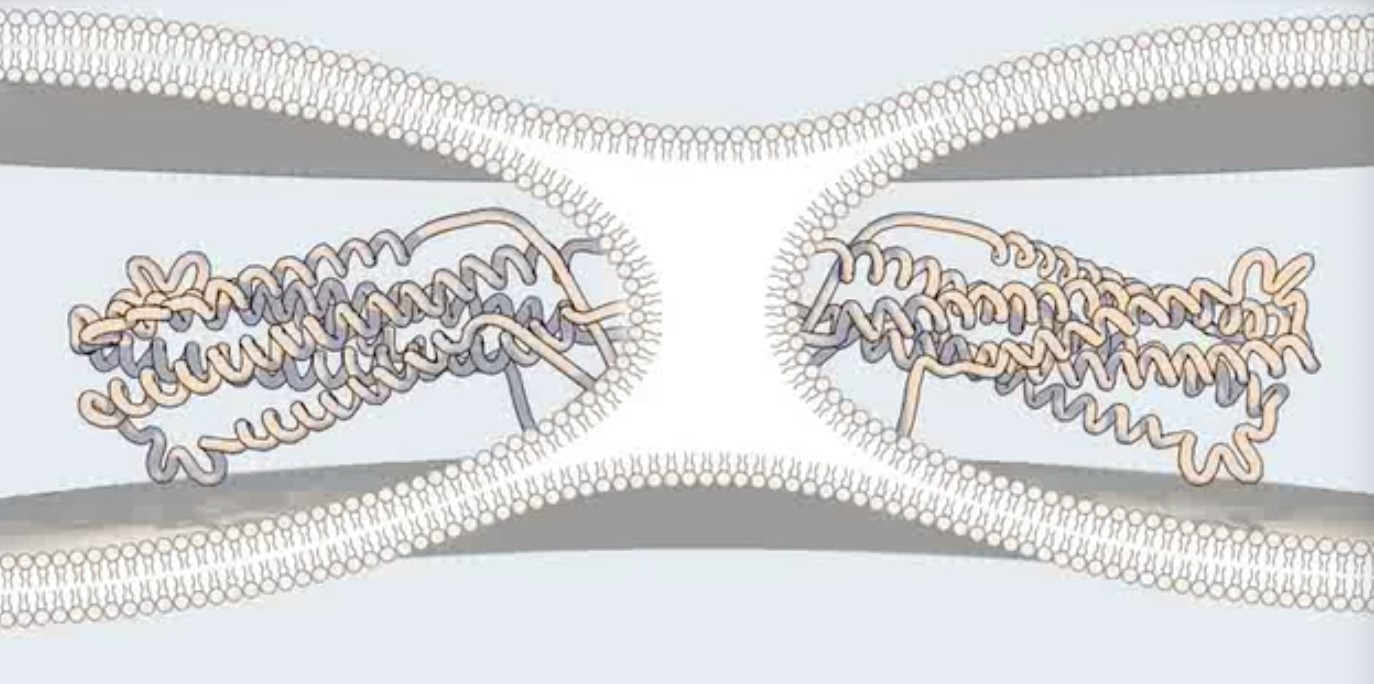
A minimum of _ are necessary to achieve membrane fusion
Two gp41 trimers

In hemifusion intermediate in HIV-1, only _ mix
outer leaflets of cell and viral membranes
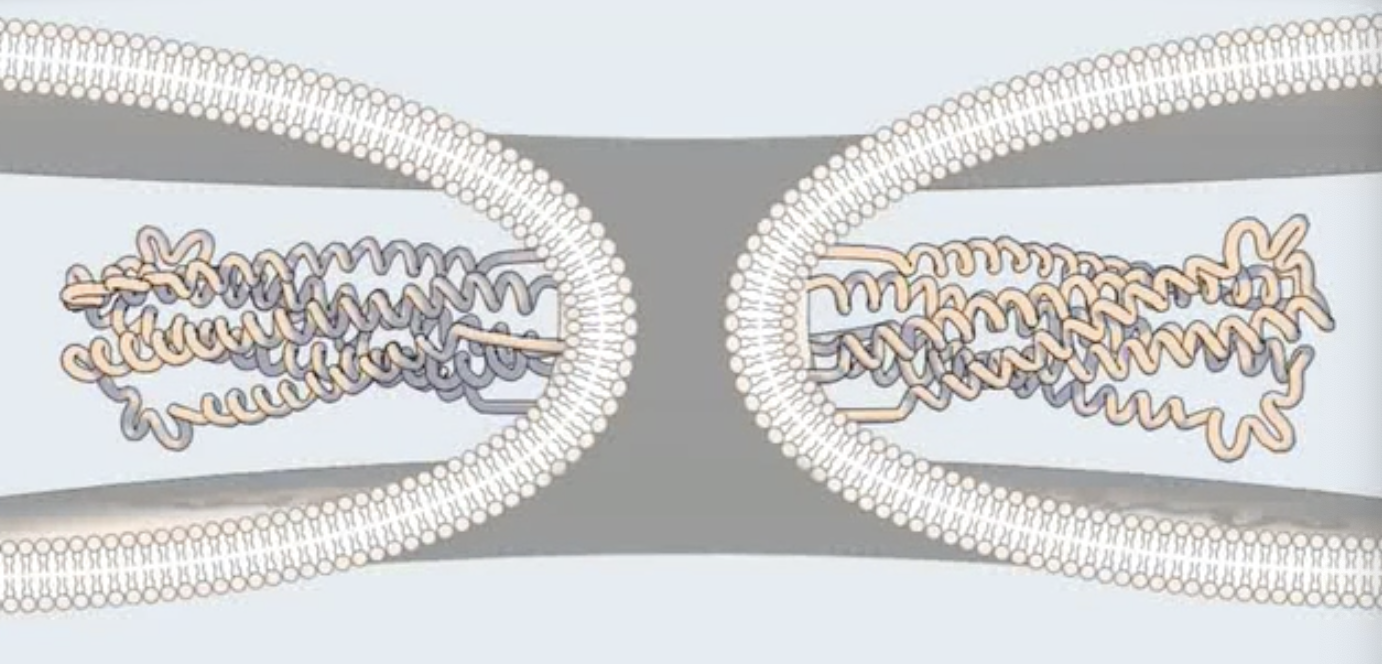
What happens during complete fusion in HIV-1?
Both inner and outer leaflets mix
Viral contents penetrate the cytosol
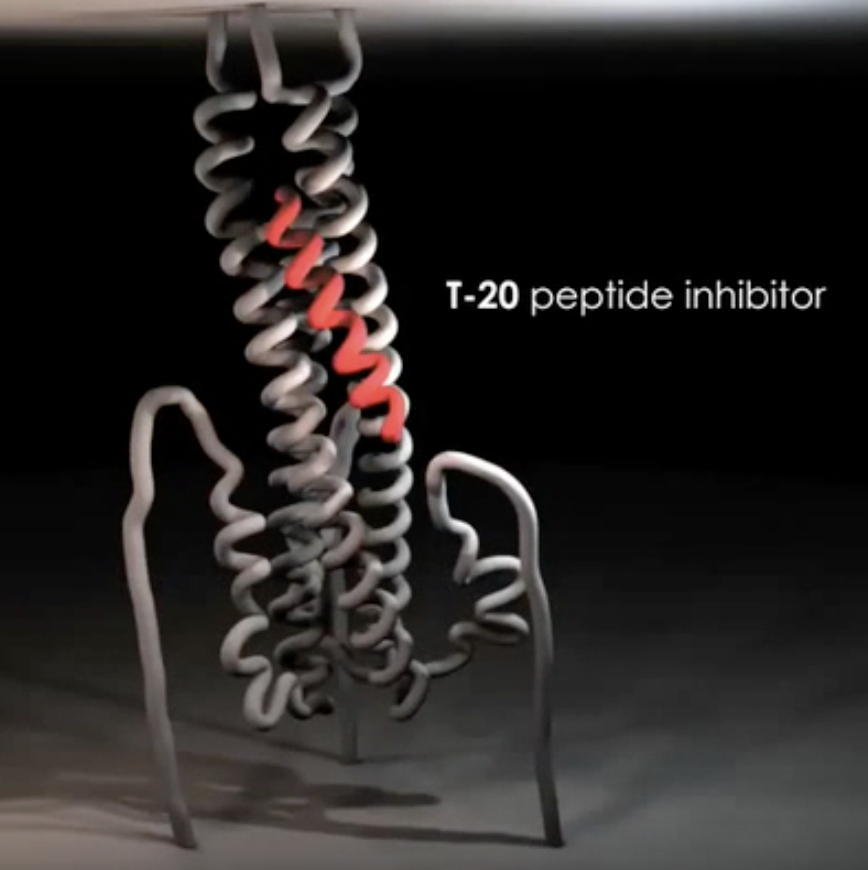
Give 1 strategy through which gp41-mediated fusion may be inhibited in HIV-1
By introducing peptide inhibitor T-20, which occupies the site alongside the trimer core and prevents the a-helical transition of the C-terminal helix
5m038 also blocks a-helical transition
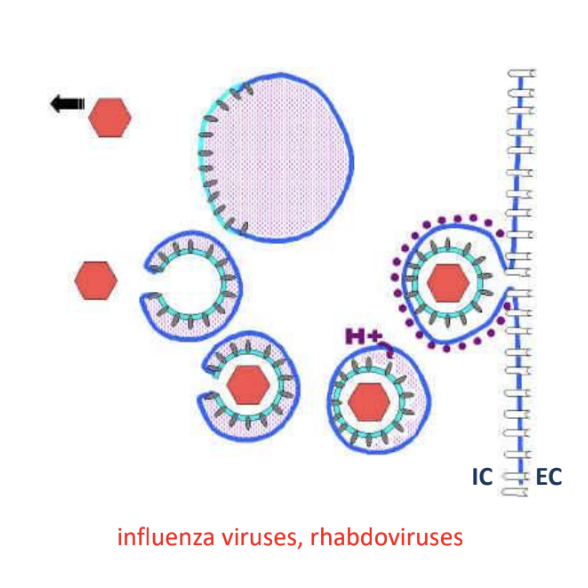
Explain what occurs in penetration via endocytosis of virion by host cell
Host cell plasma surrounds whole virion and forms a vesicle
Low pH causes virion envelope to fuse with endosome membrane of vesicle via fusion proteins
Nucleocapsid is released into cytoplasm
*Occurs in influenza viruses, rhabdoviruses
Give examples of viruses that undergo penetration via endocytosis of virion by host cell
Enveloped viruses
Influenza viruses
Rhabdoviruses
T/F: Sperm-egg fusion proteins are homologous to viral class I fusion proteins
FALSE
Sperm-egg fusion proteins are homologous to viral class II fusion proteins
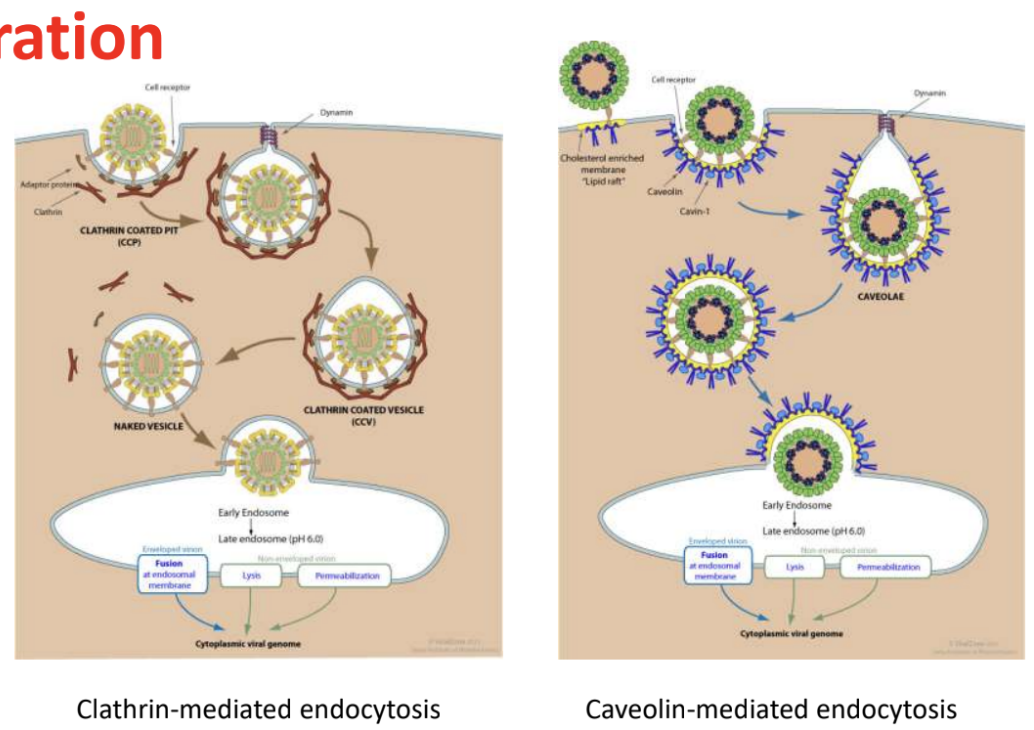
Penetration (enveloped): Clathrin-mediated vs. Caveolin-mediated endocytosis
Clathrin-mediated
Virus binds to specific host receptor, triggering formation of clathrin-coated pits
Pit buds inward, forming clathrin-coated vesicles that transport vesicle into cell
Vesicle uncoats, fuses with early endosomes, undergoes acidification (via proton pump), which triggers release of viral genome into cytoplasm
Caveolin-mediated
Virus binds to caveolae, then transported via caveolin-coated vesicles into compartments
Does not require acidification for viral genome release
For fusion to take place in influenza virus (enveloped), _
Low pH of endosome can directly alter conformation of fusion protein OR
it may activate a cell protease that cleaves the fusion protein
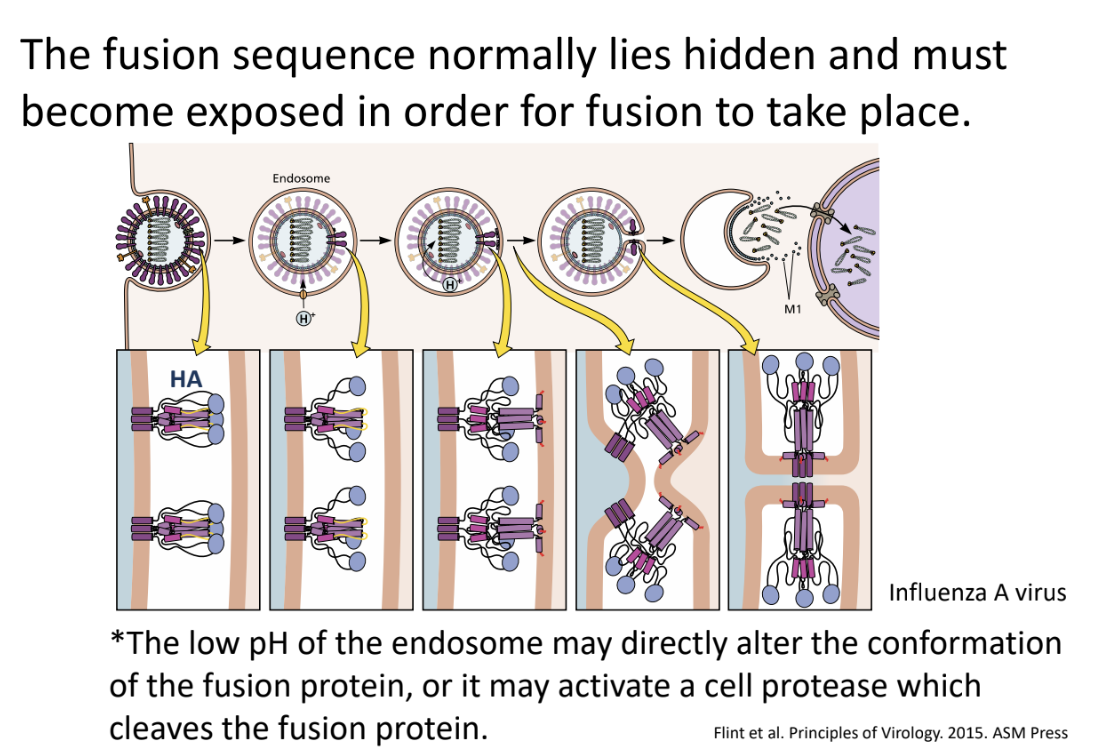
Enumerate 3 circumstances that cause conformation change in the fusion protein, allowing it to be inserted into the host cell membrane
Binding of virus to receptor gp120-CCR5 interaction (in HIV-1)
Low pH of endosome directly altering conformation (in influenza)
Activation of protease that cleaves fusion proteins (in influenza)
T/F: Hemagglutinin is a type-1 fusion protein because it is parallel to viral envelope
FALSE
Hemagglutinin is a type-1 fusion protein because it is perpendicular to viral envelope
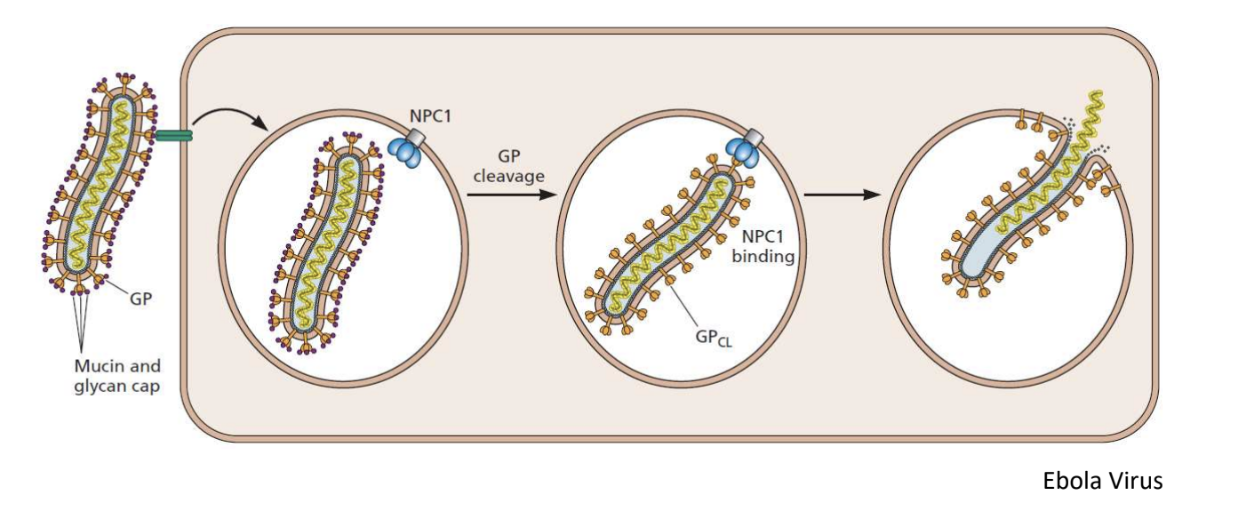
Explain penetration of ebola virus
Ebola virus doesn’t have a very specific host receptor and most likely taken up by pinocytosis
Once inside endosome, there’s cleavage of lipoproteins, exposing it to endosomal-membrane receptors, allowing fusion that releases the nucleocapsid into the cytoplasm
Different bc acidic conditions of endosome don’t change viral receptor but digests mucin cap, hiding receptor from binding to host cell receptor
*Dengue virus has similar mechanism

Envelope proteins of dengue is type _ because it is parallel to viral envelope
2
*Type 1 = perpendicular, Type 2 = parallel
_ is homologous to viral class II fusion proteins (parallel)
Sperm-egg fusion proteins
_ refer to fusion proteins essential for sexual reproduction and exoplasmic merger for plasma membranes
Fusexins
Fusexins are fusion proteins essential for _
sexual reproduction and exoplasmic mergers for plasma membranes
_ is a common origin and evolution of sexual reproduction, enveloped virus entry into cells, and somatic cell fusion
Superfamily FUSEXINS
_ refer to distinct variations within a species of virus or bacteria, classified based on differences in their surface antigens
Serotypes
Explain how immunity to 1 dengue virus (DENV) serotype can increase disease severity in humans upon subsequent infection with another DENV serotype
Immunity to 1 DENV serotype can increase disease severity in humans upon subsequent infection with another DENV serotype bc serotype cross-reactive antibodies facilitate DENV infection of myeloid cells by promoting virus entry into Fcy receptors—a process known as antibody-dependent enhancement
Why can’t hydroxychloroquine be used as treatment for COVID-19?
Plasma membrane protein facilitates fusion of host plasma membrane + viral envelope
Because cell lines they used didn’t have this plasma membrane protein, initially, it was thought that hydroxychloroquine can be used to treat COVID
However, because humans have this plasma membrane protein that provides a separate pathway for fusion of viral envelope + host cell, which cannot be blocked by hydroxychloroquine, hydroxychloroquine is then rendered ineffective against COVID
Explain 2 ways through which penetration and uncoating occurs in naked viruses
Poliovirus = partial degrading of capsid > extrusion of NA into cytoplasm
Adenovirus = endocytosis > low pH of endosomes lead to lysis/release of viral genome into cytoplasm > uncoating and delivery to nucleus
The unique shape of bacteriophages not only facilitate their attachment to host cell receptor but also _
cause ejection of their viral genome into host cell through their tail tube / channel
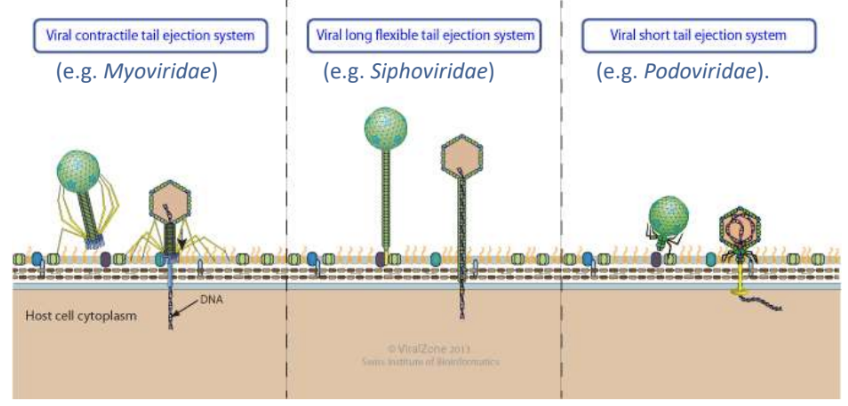
Explain what happens during viral genome ejection in naked viruses through host cell envelope via tail tube
Tailed bacterial viruses (bacteriophages) enter host cells by piercing host envelope with their tail
Delivery into cytoplasm requires ejection of viral genome from icosahedral capsid through tail tube and/or channel
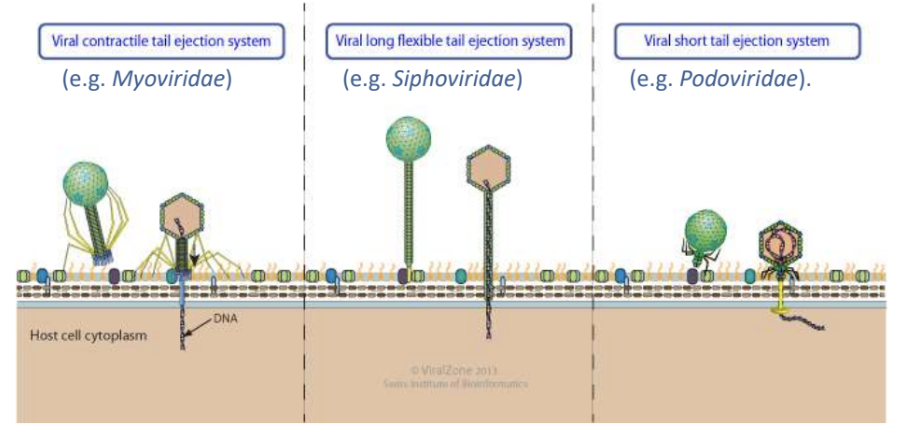
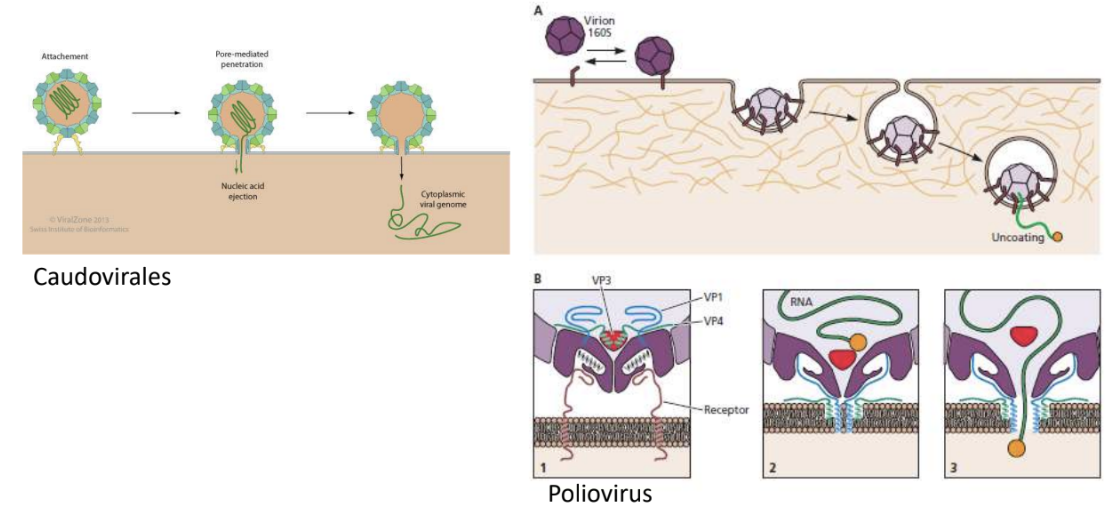
Explain 2 viruses that can do pore-mediated penetration of viral genome into host cell
Caudovirales
Poliovirus
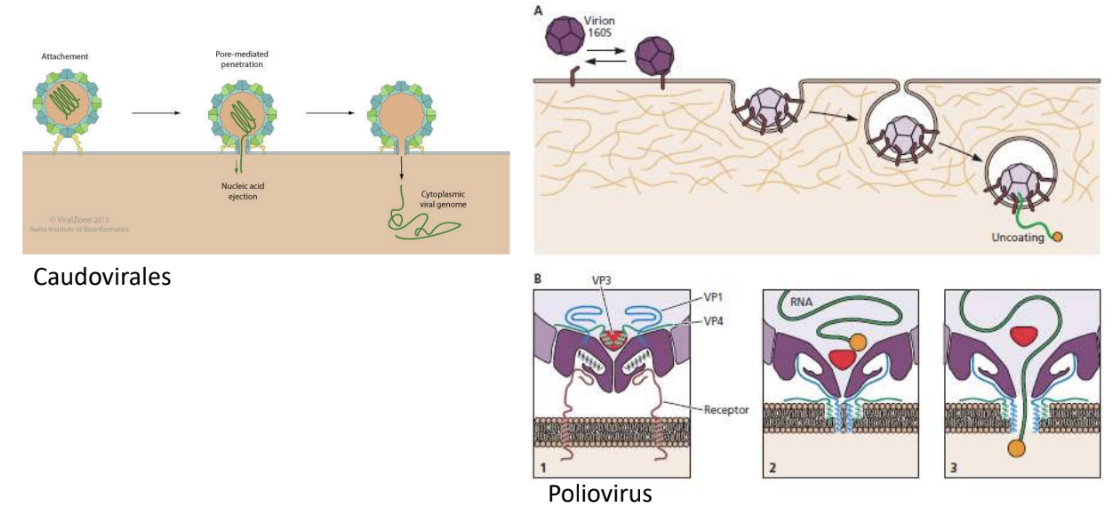
_ refers to penetration method through which viral genome is extruded into host cell without endocytosis or bypassing vesicular transport and instead forms a pore through which genome is released
Pore-mediated penetration, e.g., Caudovirales, Poliovirus
3 methods through which penetration may occur in naked viruses
Ejection of viral genome via tail tube in bacteriophages
Pore-mediated penetration
Permeabilization (break down) of host membrane
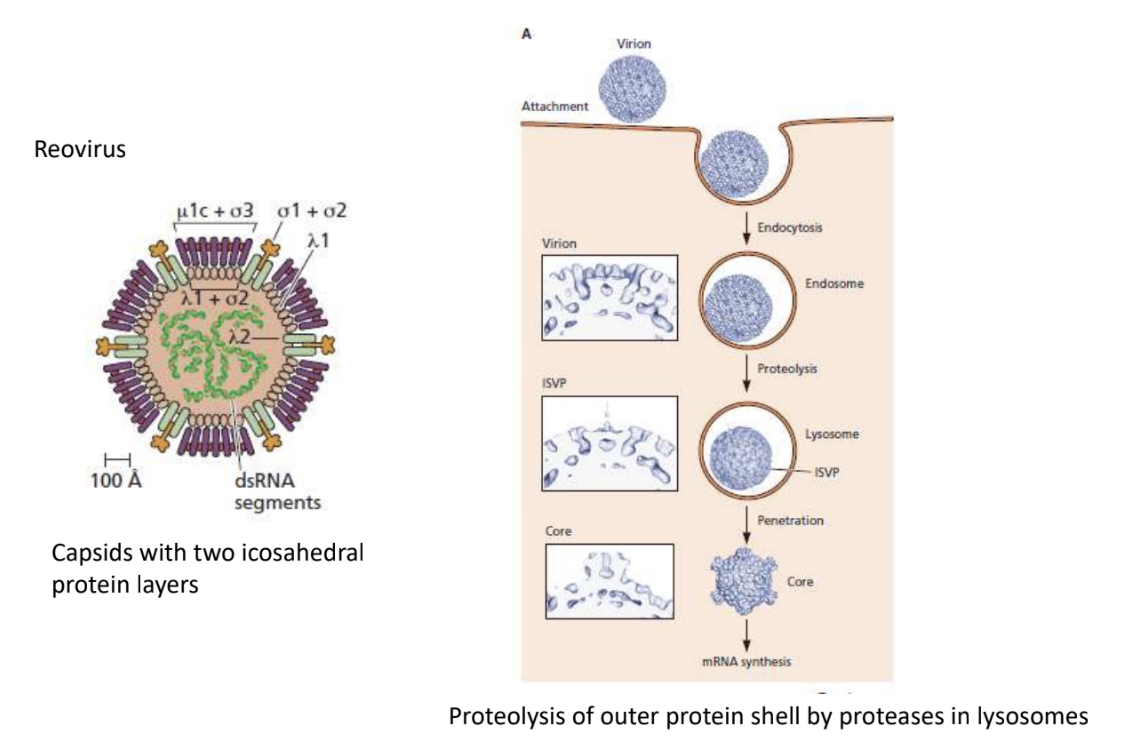
Explain unique penetration strategy of Reovirus (that increases its infectivity)
Reovirus has 2 icosahedral protein layers
Taken in via endocytosis, but lowering pH degrade only the outer coat
Virus with partially degraded single coat can then permeabilize endosomal membrane, being released into cytoplasm
It’s crucial that the virus still has 1 coat because it contains an unusual dsRNA gene that does mRNA synthesis inside to prevent host cell from recognizing presence of foreign body inside it, thus evading immune recognition