Organic Chemistry (Chp 1-3)
1/224
Earn XP
Description and Tags
Creating this from the Kaplan books. I am reading a chapter then creating flashcards from what I think is important to know for the exam
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
225 Terms
What are the steps to naming a compound?
identify parent chain
number the chain
name substituents
assign number to each substituent
complete name
Which chain is the parent chain if there is more than one of equal length connected?
the more substituted chain
Where is C1 when there is a highest order functional group?
C closest to the high-priority functional group
Where is C1 if all substituents are of equal priority?
Where the substituents have the lowest numbers
If double/triple bonds have a tie for lowest number, which takes priority?
double bond
How do you name alkanes?
replace -ane with -yl
How do you name substituents that occur multiple times?
Use prefixes: di-, tri-, tetra-
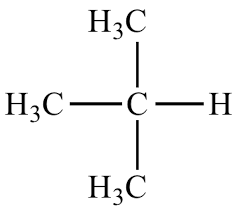
What is the common name?
t-butyl
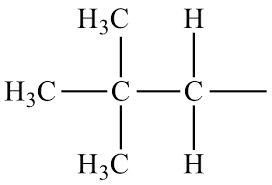
What is the common name?
neopentyl
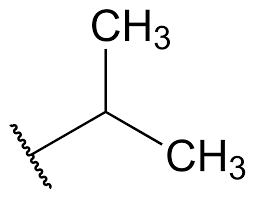
What is the common name?
isopropyl
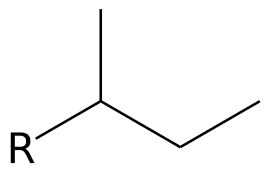
What is the common name?
sec-butyl
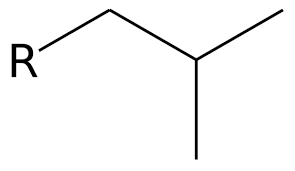
What is the common name?
isobutyl
How do you number the substituents?
It is the same as the C they are attached to
How do you put the name together?
substituents are placed in abc order
number goes in front of substituent
numbers are separated by commas
words are separated by dashes
finish with parent chain name including highest functional group
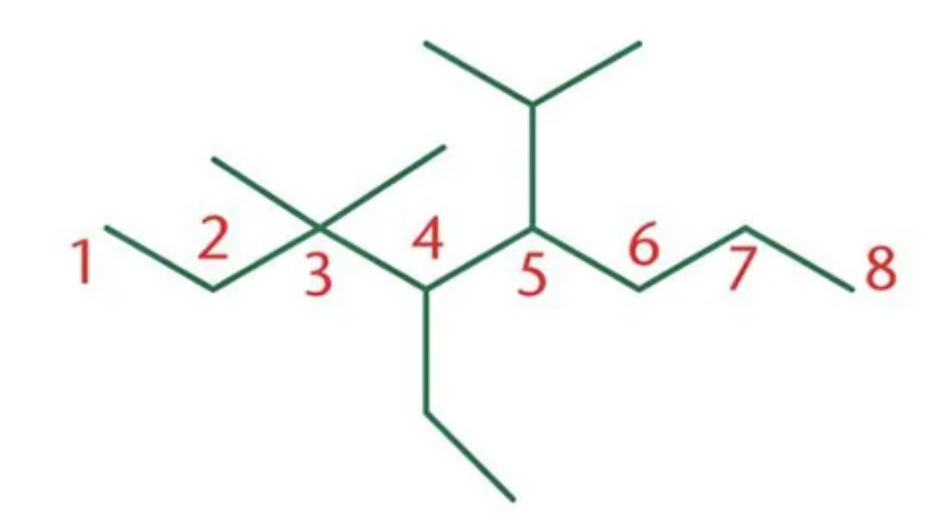
What is the name of this compound?
4-ethyl-5-isopropyl-3,3-dimethyloctane
What is a hydrocarbon?
molecule that only contains C and H
What is an alcohol?
molecule that contains at least one -OH group
What is the general formula for hydrocarbons?
CnH(2n+2)
How do you name alkyl halides?
prefix: fluoro-, chloro-, bromo-, iodo-
How do you name double bonds?
replace -ane with -ene
How do you name triple bonds?
replace -ane with -yne
Which number in the double/triple bond gets added to the name?
whichever is lower
What is the alkane nomenclature for 1-8C chains?
methane
ethane
propane
butane
pentane
hexane
heptane
octane
How do you name alcohols?
replace -e with -ol
What is the prefix for alcohols if they’re not the highest functional group?
hydroxy-
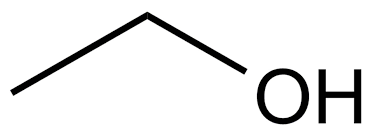
What is the name?
ethanol
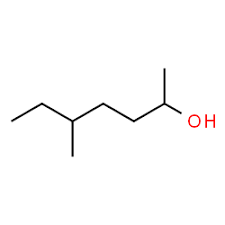
What is the name?
5-methyl-2-heptanol
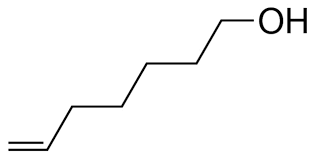
What is the name?
6-hepten-1-ol
What is the common name formula for alcohols?
alkyl group + alcohol
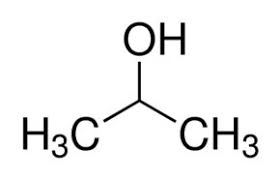
What is the common name?
isopropyl alcohol
How do you name an alcohol with 2 hydroxyl groups?
replace -ol with -diol and add both numbers to front
What is a geminal diol?
alcohol with two hydroxyl groups on the came C
What is a vicinal diol?
alcohol with two hydroxyl groups on adjacent Cs
What is another name for geminal diols?
hydrates
Why are geminal diols not commonly seen?
they spontaneously dehydrate to create a carbonyl compound (has C=O)
What’s the difference between aldehydes and ketones?
aldehydes are on terminal C while ketones are on a C in the middle of the chain
How do you name an aldehyde?
replace -e with -al
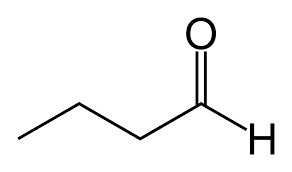
What is the name?
butanal
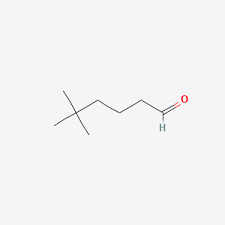
What is the name?
5,5-dimethylhexanal
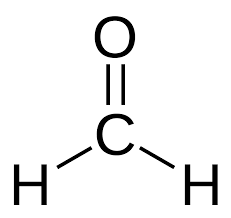
What is the common name?
formaldehyde
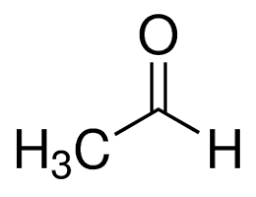
What is the common name?
acetaldehyde
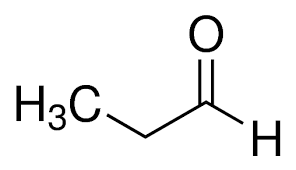
What is the common name?
propionaldehyde
How do you name a ketone?
replace -e with -one
What is the common name formula for ketones?
alkyl groups in ABC order + ketone
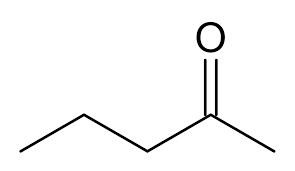
What is the name?
2-pentanone
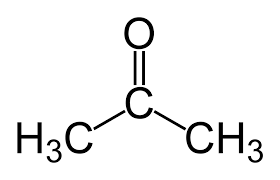
What is the IUPAC name
IUPAC: 2-propanone
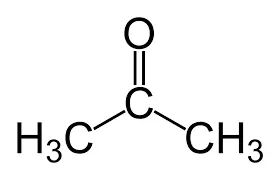
What is the common name using the formula?
dimethylketone
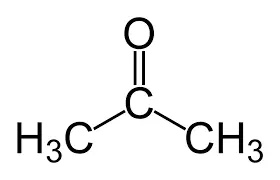
What is the common name?
acetone
What is the prefix for aldehydes if not highest priority?
oxo-
What is the prefix for ketones if not highest priority?
keto- or oxo-
With carbonyls, how else may the adjacent Cs be named?
greek letters
alpha ⍺ → beta β → gamma 𝛄 → delta 𝛅
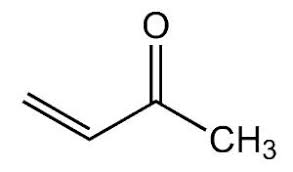
What is the IUPAC and common name?
IUPAC: 3-butene-2-one
Common: methylvinylketone
What is a Carboxylic Acid?
terminal COOH
How do you name carboxylic acids?
replace -e with -oic acid
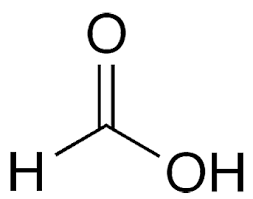
What is the common name?
formic acid
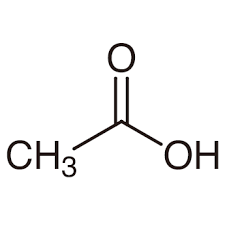
What is the common name?
acetic acid
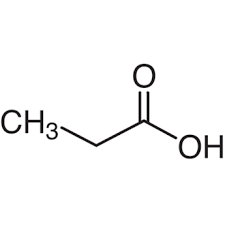
What is the common name?
propionic acid
What is an ester?
COOR
What is the R group in an ester?
hydrocarbon chain
How do you name an ester?
first term: alkyl name of R group
second term: parent acid with -oate instead of -oic acid
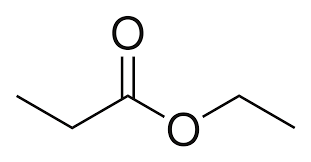
What is the name?
ethyl propanoate
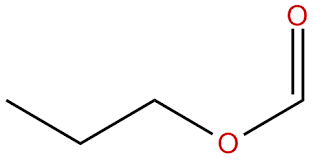
What is the name?
propyl methanoate
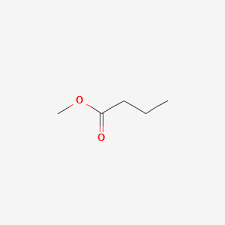
What is the name?
methyl butanoate
What is an amide?
CONR
How many R groups can there be in an amide?
up to 3
How do you number the R groups in an amide?
N instead of a number
How do you name an amide?
first term: alkyl groups attached to N
second term: parent acid with -amide suffix
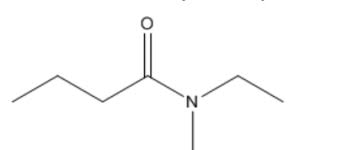
What is the name?
N-ethyl-N-methylbutanamide
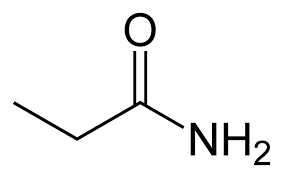
What is the name?
propanamide
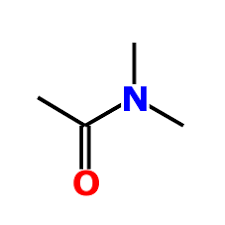
What is the name?
N,N-dimethylethanamide
What is an anhydride?
2 carboxylic acids attached with a water removed
What is the structure of most anhydrides?
cyclic
How do you name a symmetric anhydride?
replace -acid with -anhydride
How do you name an asymmetric anyhydride?
name both acids followed by anhydride
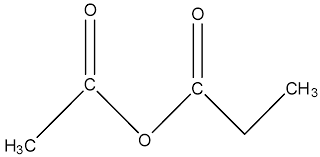
What is the name?
ethanoic propanoic anhydride
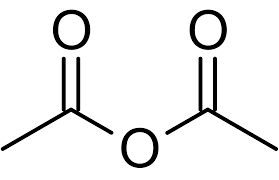
What is the IUPAC and common name?
IUPAC: ethanoic anhydride
Common: acetic anhydride
What is the priority list of functional groups from highest to lowest?
carboxylic acid
anhydride
ester
amide
aldehyde
ketone
alcohol
alkene
alkyne
alkane
What is the prefix and suffix of carboxylic acids?
prefix: carboxy-
suffix: -oic acid
What is the prefix and suffix of anhydrides?
prefix: alkyloxycarbonyl-
suffix: -anhydride
What is the prefix and suffix of esters?
prefix: alkoxycarbonyl-
suffix: -oate
What is the prefix and suffix of amides?
prefix: carbamoyl- or amido-
suffix: -amide
What is the prefix and suffix of aldehydes?
prefix: oxo-
suffix: -al
What is the prefix and suffix of ketones?
prefix: oxo- or keto-
suffix: -one
What is the prefix and suffix of alcohols?
prefix: hydroxy-
suffix: -ol
What is the prefix and suffix of alkenes?
prefix: alkenyl-
Suffix: -ene
What is the prefix and suffix of alkynes?
prefix: alkenyl-
suffix: -yl
What is the prefix and suffix of alkanes?
prefix: alkyl-
suffix: -ane
What is a structural isomer?
Two molecules that have the same formula
What must be the same for structural isomers?
Molecular weight
For structural isomers, are chemical properties same or different?
different
For structural isomers, are physical properties same or different?
Different
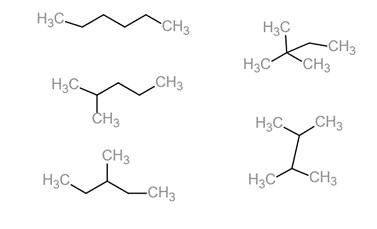
What kind of isomer are these?
Structural
What are stereoisomers?
share same atomic connectivity but differ in 3D orientation
What are conformational isomers?
molecules that differ in rotation around a single bond
What are configurational isomers?
molecules that can only be interconverted by breaking a bond
What is a good way to visualize conformational isomers?
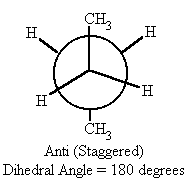
Newman Projections
Where is a Newman projection visualized?
along line extending through C-C bond axis
What conformation is the lowest energy-state for straight-chain conformational isomers?
anti conformation
What is staggered conformation?
No overlap of atoms along line of sight
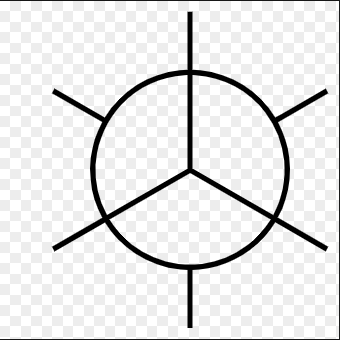
What is Anti Conformation?
Two largest groups are in same plane but on opposite sides