Bio Questions
1/257
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
258 Terms
What are all living organisms composed of?
Cells
How big are cells?
Most are 10uM to 100um in size but can get as long as 1 meter
What is the log of 0.001?
-3
How do cells arise?
Via division of preexisting cells
What is the genetic material of all cells?
DNA
What s a nucleoid?
Single, circular molecule of DNA found in a prokaryote cell
What is a nucleus?
Where DNA is stored in Eukaryotes
What does the plasma membrane do?
It’s a boarder between the inner cell and outer environment that controls the movement of materials into and out of the cell
What are the two major types of cells?
Prokaryotes and eukaryotes
What define a prokaryote?
It is without a nucleus
What defines a eukaryote?
It has a nucleus
How are prokaryotic and eukaryotic cells similar?
They both have DNA, a plasma membrane, cytoplasm, ribosomes
How are prokaryotic and eukaryotic cells different?
Prokaryotes lack interior organization and don’t have bound organelles such as the nucleus or mitochondria, they have DNA as a nucleoid and they all have a cell wall on top of the membrane. Eukaryotes have their DNA packaged into chromosomes in the nucleus and only plant cells have a cell wall.
What is a fact?
An observation
What is a law?
A description that explains the behavior of a system, but not why it behaves in this way
What is a theory?
A well-substantiated explanation of facts that describes how something happens; used to make predictions
What is a hypothesis?
A question that can have no answer and is tested by experiments and observation; it can almost never be proven
What is an atom?
A basic unit of matter than contains protons, electrons and neutrons
What is a proton?
A positively charged particle
What is a neutron?
A particle with no charge
What is an electron?
A negatively charged partical
How do you calculate atomic mass?
Number of neutrons + the number of protons
How do you calculate the charge of an atom?
The number of protons - the number of electrons
What is an isotope?
An atom that has an abnormal number of neutrons
Where are protons, neutrons and electrons each found?
Protons and neutrons are found in the nucleus while electrons orbit the nucleus
What is a molecule?
A collection of atoms held together by covalent chemical bonds
What is the difference between an atom and a molecule?
An atom is a structure made up of protons, neutrons and electrons and bonds with other atoms via covalent bonds to form molecules while molecules are collections of atoms
How does an electron impact the chemistry of an atom?
It helps determines which atoms are more likely to bond to each other and the number of electron in relation to protons determines the charge of the atom
How does a neutron impact the chemistry of an atom?
It determines the isotope and atomic mass
How does a proton impact the chemistry of an atom?
Protons determine the electronegativity of atom which determines bond type
What are orbitals?
Areas where electrons have a high probability of being localized, different orbitals are at different energy levels
What are shells?
Orbitals
How many electrons go in each of the first three shells?
1st: 2; 2nd: 8; 3rd: 8
What are valence electrons?
Electrons in the outermost shell which help determine the chemical bonds atoms form with each other
How are covalent bonds formed?
When atoms share a pair valence electrons
What do Lewis Structures show?
Valence electrons
List the bond types from strongest to weakest
Covalent, ionic and hydrogen, hydrophobic interaction, then Van der Waals
How does electronegativity relate to hydrogen bonds and hydrophobic interactions?
Hydrophobic compounds have nonpolar covalent bonds meaning they cannot form hydrogen bonds with other molecules and they are usually forced into hydrophobic interactions while water molecules have polar covalent bonds which allow them to form hydrogen bonds with some other molecules with polar covalent bonds due to partial charges and they push nonpolar compounds together
What do Joules and calories measure?
Joule is a unit of energy where ~4.2J=1 calorie which is the amount of energy that is required to raise 1 gram of water by 1 degree
At what temperature do covalent bonds break?
Over 3000
Does cooking food break covalent bonds?
No
What is cohesion?
It arises because of the attraction between H2O molecules
What effects does cohesion have on the behavior of water?
It allows for surface tension, hydrophobic exclusion, and the high heat of vaporization
What is adhesion in the context of water, and what impact does it have on its behavior?
It is where water molecules are attracted to other molecules and it allows for water to dissolve substances, make things wet and for capillary action
How do hydrogen bonds arise?
It is because of an electrostatic attraction that occurs when a hydrogen atom with a partial positive charge is attracted to an atom with a partial negative charge which arise due to electronegativity
What is a partial charge?
It is where electrons are more attracted to one atoms of the molecule due to electronegativity giving that atom a partial negative charge and other atoms a partial positive charge
Are partial charges present in nonpolar covalent bonds?
No
When hydrogen bonding occurs with a phosphate ion, what atom does the H in water interacts with: O or P?
O because it is more electo
How do ions form?
When an atom gains or loses electrons
Are ionic bonds weaker than polar covalent bonds?
Yes
What makes something an acid? What is the corresponding pH?
It is a molecule that donates protons to water meaning the concentrations of protons ([H+]) increases. pH<7
What makes something a base? What is the corresponding pH?
It is a molecule that accepts protons from water leaving a decreased concentrations of protons ([H+]) and an increased concentration of hydroxls [OH-]. pH>7
What is the charge on amino acids at pH 7 and 12?
pH 7: H+ and pH: N/A
What is the charge on carboxyl groups at pH 0 and 7?
pH 0: N/A and pH 7: -
Why do low and high pHs disrupt the structure of organic molecules like proteins?
Low pH’s cause protons [H+] to bond to open negative charges and high pH’s cause positively charged molecules to loose their extra proton; this means these molecules can no longer for ionic attraction
Are hydrophobic molecules attracted to each other?
They are pushed out from water because they cannot form hydrogen bonds which forces them together into hydrophobic bonds; hydrophobic bonds do not occur because hydrophobic molecules are attracted to each other
What are London dispersion forces?
They are attractions between molecule due to instaneous dipoles; very weak
What electronegativity is necessary to create nonpolar covalent bonds?
A small difference in electronegativity between atoms
What electronegativity is necessary to create polar covalent bonds?
A larger difference in electronegativity between atoms
What electronegativity is necessary to create ionic bonds?
A large difference in electronegativity between atoms because electrons are transferred from one atom to another
What is the concentration of protons in pH 7?
1 × 10^7
What is 1 millimolar (mM)?
1 × 10^-3
What is one micromolar (uM)?
1 × 10-6
What is 1 nanomolar (1 nM)?
1 × 10^9
What is the equation for pH?
pH = -log10([H+])
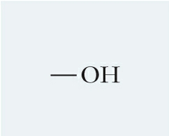
What is this functional group and describe it
Hydroxyl, a partially charged polar molecule that forms H-bonds and makes other molecules soluble in water (hydrophilic)
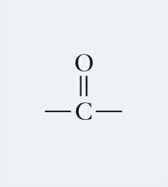
What is this functional group and describe it?
Carbonyl, a partially charged polar molecule that forms H-bonds and makes other molecules soluble in water
What is a ketone?
A carbonyl that is attached to 2 carbon atoms
What is a aldehyde?
A carbonyl attached to at least one hydrogen atom
What is an ester?
Carbonyls that have an oxygen and carbon attached
What is an amide?
Carbonyls with a nitrogen attached
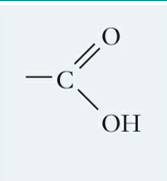
What is this functional group and describe it?
Carboxyl, a polar molecule that forms H-bonds and makes other molecules soluble in water; at pH=7 it loses a proton to become negatively charged allowing it to have ionic interactions
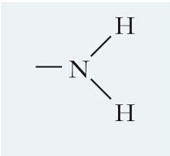
What is this functional group and describe it?
Amino, a polar molecule that forms H-bonds and makes other molecules soluble in water; at pH=7 it gains a proton to become positively charged allowing it to have ionic interactions
What functional group do proteins begin with? What is this called?
Amino group is at the N terminus
What functional group do proteins end with? What is this called?
Carboxyl group is at the C-terminus
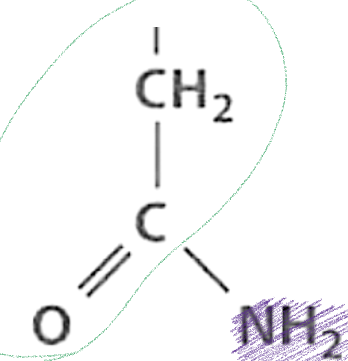
What is this functional group and describe it?
Amides, a molecule that contains a amino and carbonyl group; since the carbonyl group attracts electrons, protons no longer bind to the amino group so it cannot ionize but does form H-bonds
What functional group helps in stabilization of protein structure by forming covalent cross-links?
Sulfhydryl groups
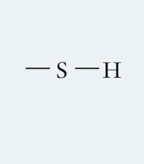
What is this functional group and describe it?
Sulfhydryl, two bonds can react to form S-S (disulfide bonds) to stabilize proteins; they exhibit little hydrogen bonding and are less soluble in water
What functional group makes peptide bonds soluble?
Carbonyl groups
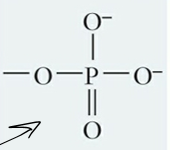
What is this functional group and describe it?
Phosphate, a charged polar molecule that forms H-bonds and makes other molecules soluble in water
What happens to phosphate groups in ATP at pH 7?
It donates hydrogen atoms (protons) that are bonded to available oxygen atoms making ATP a weak acid and then giving those atoms a negative charge allowing them to form ionic interactions
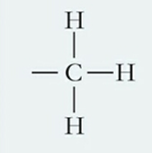
What is this functional group and describe it?
Methyl, a nonpolar, hydrophobic molecule that forms no H-bonds and is often found in lipids
What element makes up the most of the dry mass of a cell?
Carbon
Why is ethanol soluble in water?
Because the inclusion of the hydroxyl group makes it water soluble
How does DNA differ from RNA?
RNA has one more hydroxyl group than DNA at 2’ while DNA only has a hydrogen atom there
In which macromolecule is carboxyl groups found?
Amino acids
What are the five parts of an amino acid?
alpha carbon (central carbon), amino group (-NH2), carboxyl group (-COOH), hydrogen atom and R group/side chain
What do carboxyl groups do in proteins?
They stop the amino group from gaining an extra proton in basic solutions
What is the name of the bonds that holds amino acids together?
Peptide bonds
What is the charge on anino and carboxyl group at pH 0?
The amino group has a single positive charge, so it is positively charged
What is the charge on amino and carboxyl group at pH 6?
The amino group has a single positive charge and the carboxyl group has a single negative charge so the molecule is neutral
What is the charge on anamino and carboxyl group at pH 11?
The carboxyl group has a single negative charge so the molecule is negatively charged
Howdo di-sulfhydryl bonds stabilize the protein structure?
By covalently cross-linking different parts of the protein together
In what molecules is phosphate found?
ATP and nucleic acids
What is the charge on phosphate at pH 7?
It is negatively charged
Which functional groups form hydrogen bonds?
Hydroxyls, carbonyl, carboxyl (at pH values<7), amino acids (at pH values<7), amides on the amino group and phosphates
What are the four major classes of macromolecules?
Carbohydrates, nucleic acids, proteins and lipids
Which macromolecules are polymers?
Carbohydrates, nucleic acids and proteins
What is a polymer?
A long molecule consisting of many similar subunits called monomers linked by covalent bonds
What does lipid mean?
Soluble in non-polar solvents