BLD 204 Final
1/241
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
242 Terms
• Describe the cell and cellular organelles. Know the functions of the membrane and the organelles discussed in class.
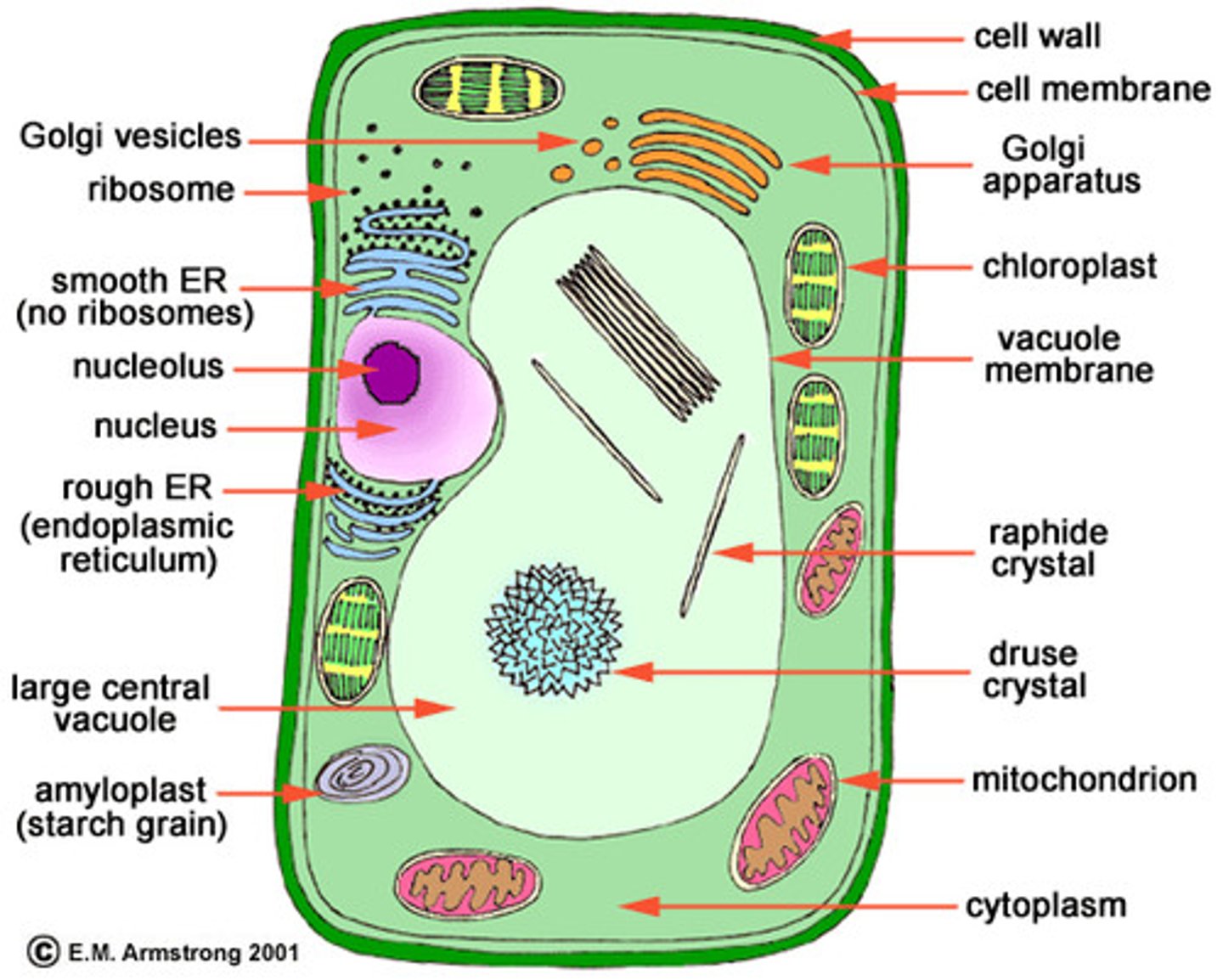
What is Mitochondria
Powerhouse of the cell, organelle that is the site of ATP (energy) production
what is a Lysosome
membranous sac of hydrolytic enzymes that can digest macromolecules. Garbage collectors breakdown cellular debris.
What is the nucleus?
controls and regulates the activities of the cell and carries genes
what is smooth ER
lipid synthesis, detoxification, carbohydrate metabolism, It lacks ribo
What is the rough ER?
membrane enclosed passageway for transporting materials. Studded with ribosomes, responsible for protein synthesis, and sends them to the golgi
what is golgi complex
receives vesicle, as proteins move through golgi body the protein are customized into forms the cell can use, by folding the proteins or adding materials. post office of the cell
What is a microtubule?
hollow structures made up of proteins called Tubulins and form Centrioles. supports cell and aids in cell movement
What is a microfilament?
threadlike structures made of protein and Produce a flexible framework for the cell. enable motility, contraction, and intracellular transport
Know the major causes of cell injury and the major biochemical mechanisms of cell injury.
- hypoxia
- toxins
- genetic problems
- physical damage
- irradiation
- infection
- immune system reactions
- nutritional causes of disease
- aging
what is hypoxia and the potential causes
deficiency in the amount of oxygen reaching the tissues
causes: ischemia, inadequate blood oxygenation (not enough hemoglobin), lower blood oxygen carrying capacity (carbon monoxide)
What is ischemia?
an inadequate blood supply to an organ or part of the body, especially the heart muscles.
what are toxins and possible causes
substances cause damage to cells, often through membrane damage
causes:
too much of a good thing (like caffeine or sugar), poisons, ethanol, air pollutants, and asbestos
what are genetic problems and possible causes
congenital (born with it) or later mutations
causes:
inherited defects, accumulation of damaged DNA or proteins
what are physical damage and possible causes
outside action by the physical world
causes:
temperature, mechanical damage, electric shock, barometric pressure, irradiation
irradiation:
categorized with physical damage: electromagnetic or particulate
danger from radiation is based on strength of dose and duration of dose and what part of the body is exposed
what are infection and possible causes
causes: infection by viruses, fungi, protozoa, other parasites, and bacteria cause various types of damage
* toxins are especially likely culprits with cellular injury from bacteria
what are the causes a immune system reaction
causes: autoimmunity, allergy, chronic inflammation
what are nutritional causes
causes: calorie deficiency, specific vitamin deficiencies, obesity, improper nutrition
example: a glycogen-replete hepatocyte will survive ischemia better than 1 that has just burned its last glucose molecule
what is aging and how does it cause damage
overall aging or senescence results in cells which are less able to respond to damage
what areas cause the most harm to if injured
mitochondria, calcium homeostasis, membrane (cell or lysosomes or mitochondria), DNA, and protein folding
When does ATP depletion occur? And where is ATP made?
This occurs when hypoxia, lack of nutrients, damage to the mitochondria, and some toxin
The major source of ATP is oxidative phosphorylation in the mitochondria.
What happens to the cell when ATP depletion occurs?
Na+ pumps dont function -> no osmotic balance leads to -> cellular swelling/ER dilation
Increase in anaerobic glycolysis to try to generate some ATP
Ca++ pumps fail: Ca++ influx
Protein synthesis is disrupted
What happens in mitochondrial damage? how can mitochondria be damaged?
- atp depletion
- ROS increase: oxidative stress
- increased mitochondrial membrane permeability
- apoptosis
can be damaged:
hypoxia, toxins, radiation
What is Ca++ influx?
- Ca++ influx is when the Ca++ pump fails.
- cellular Ca++ is mostly sequestered mitochondria and ER
- Cytosolic concentration are up to 10000x less than extracellular
Compare and contrast Ca2+ influx and ROS and how they affect membrane, proteins, DNA, atp
1: Ca2+ influx and 2: ROS
Mem:
1. activates phospholipases
2. lipid peroxidation
Proteins:
1. proteases 2. misfolding or damage
DNA:
1. endonucleases 2. mutations due to chemistry
ATP:
1. ATPase
what causes membrane damage/permeability increases? what are the Biochemical explanations?
Causes: bacterial toxins, complement, chemical toxins, physical damage, reduced vascular supply
Biochemical explanations:
- phospholipids: decrease synthesis or increase destruction
-ROS
-Damaged cytoskeleton
- lipid breakdown products act as detergents
The four major types of necrosis and where you would find them
- Coagulative necrosis: characteristic of infarcts in solid organs (except brain)
- fat necrosis: high fat areas (breast) or by release of activated pancreatic enzymes (acute pancreatitis)
- caseous necrosis: fragmented or lysed cells seen, "cheese like", common in granulomas (TB), lung
- liquefactive necrosis : seen in CNS in infarcts (inadequate blood supply to the affected area)
- fibrinoid necrosis: appearance is fibrin-like, appears in small blood vessels
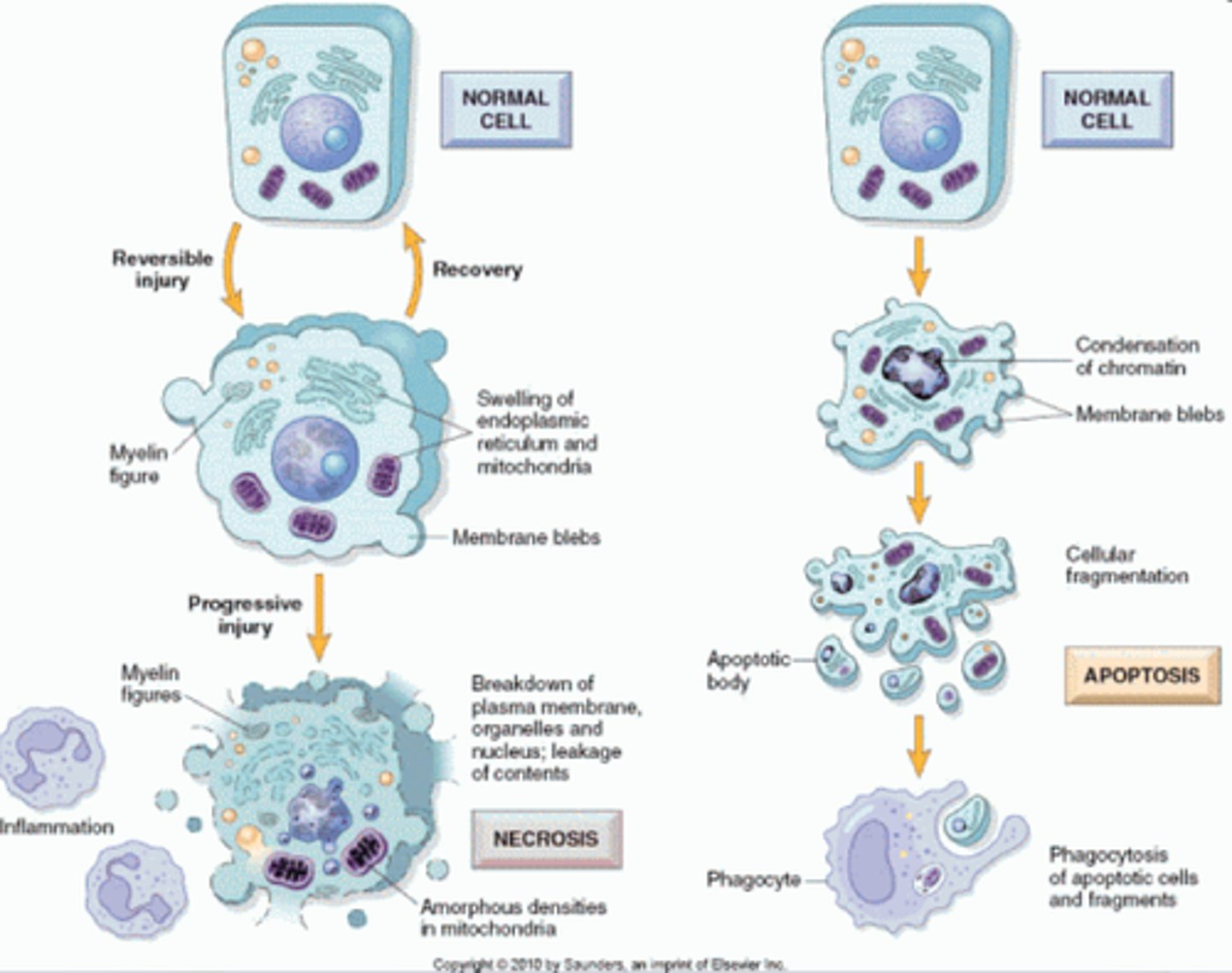
• Compare and contrast apoptosis and necrosis, include the outcomes, how the cell and nucleus break down
draw diagram
Apop:
- cell controlled cell death
- can occur under normal physiological conditions or pathologic conditions
- gets rid of cells WITHOUT ELICITING AN IMMUNE RESPONSE to them
- begins with condensation of the chromatin and blebbing of the membrane : with that the cell beings to fragment, this creates apoptotic bodies that contain cellular contents so they aren't spilled into the open: phagocytes engulf apoptotic bodies which send out "eat me" signals and degrade them NO inflammation
Necrosis:
cell death from irreversible damage.
- Loss of membrane integrity
- leakage of lysosomes and cell contents
- cell dissolution often by enzymes released from the cells themselves, no control, autolysis
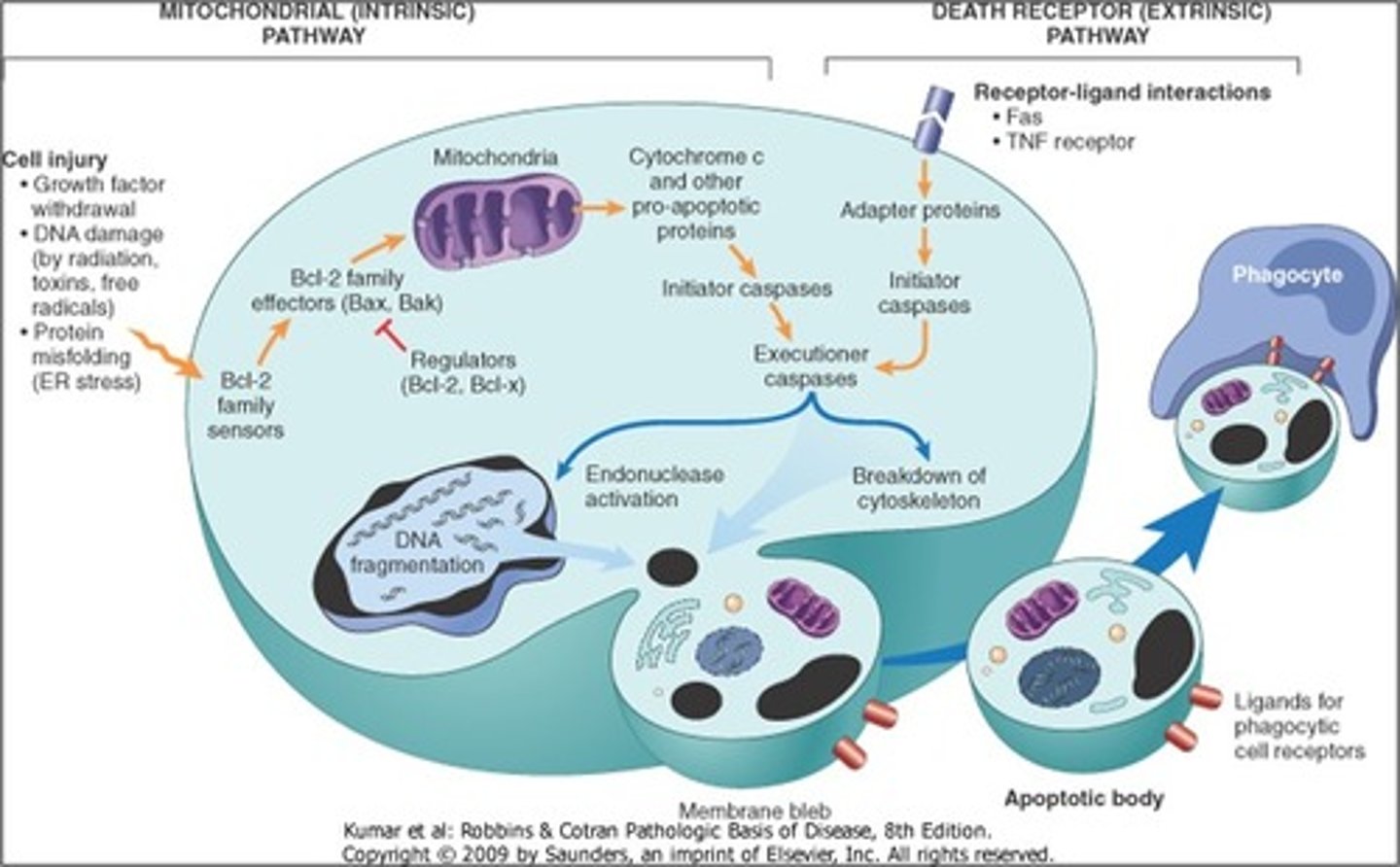
intrinsic and extrinsic apoptotic pathways.
draw diagram
When does apoptosis occur normally? vs non normal
Normal:
- embryogenesis (development of a fetus)
- cells that have served their purpose (aging out)
- self- reactive lymphocytes (if lymph react to you they go down apop.)
- induced by cytotoxic T lymphocytes
- management of proliferation cell population
- breakdown of hormone dependent tissues
non normal:
DNA damage, Misfolded proteins, viral infections, atrophy of glands after duct obstruction, or oxidative stress
• The five cardinal signs of inflammation and how they happen
- heat (vasodilation)
- redness (vasodilation)
- swelling (vasopermeability)
- pain (edema and vasodilation)
- loss of function
• What's the goal of inflammation?
Goal is to eliminate the cause and damaged tissues and initiate repair
- meant to be protective
• When is inflammation a problem?
- when the inflammatory response is severe
- when the response is prolonged
- when the response is inappropriate
• What's the difference between acute and chronic inflammation?
acute: is fast strong response
chronic: is long weak response
Acute inflammation sum
two major components:
- vascular changes (increased permeability and dilation)
- cellular response
• Know the major cells involved in inflammation and how and when they function in the process **
1st: Edema
2nd: WBC mainly neutrophils are first to migrate to sit of infection.
3rd: macrophages arrive. the cells phagocytose foreign substances and generate proteins and other molecules that function in inflammatory process
• Know the cytokines involved in inflammation and healing and their functions **
IL-1:
- pro-inflammatory
- source: Macrophages, B cells, DCs
- function: Pyrogenic, pro-inflammatory, proliferation and differentiation, BM cell proliferation
IL-6:
- Pro-inflammatory
- Sources: Th Cells, macrophages, fibroblasts
- function: B-cell differentiation
TNF - alpha:
- Pro-inflammatory
- source: Macrophages
- function: Phagocyte cell activation, endotoxic shock
Anti-inflam:
IL-10:
- Anti-inflammatory
- source: T cells, B cells, macrophages
- function: Inhibits cytokine production and mononuclear cell function
IL-12: Anti-inflammatory
function: Activates NK cells, phagocyte cell activation,
TGF-β: Anti-inflammatory
- functions: Inhibits T and B cell proliferation, inhibits hematopoiesis, promote wound healing
• Describe WBC recruitment
1. margination and rolling
2. adhesion to endothelium
3. transmigration through the endothelium extravasation
4. migration into interstitial tissue
Four major steps
- margination and rolling:
1. macrophages use toll-like receptors to detect microbes. then they phagocytize them
2. they release procytokine (TNF, IL-1) and chemokines. The cytokines serve as an inflammatory response signal and changes the gene expression of the endothelium
3. the endothelium now have P-selectin and E-selectin receptors
4. the leukocytes come in with low affinity integrin (means not ready to bind)
5. SLX protein and selectins receptors bind first to the endothelium surface, chemokine then binds to proteoglycan this causes the change in affinity of the incoming WBC surface (or integrin protein)
6. the integrin on incoming now binds to ligand CAM-1
- adhesion to endothelium
7. the binding to the high affinity integrin stops the leukocyte from rolling
8. this allows the cell to change gene expression again which both the cell endothelium start to express the protein PECAM-1 or CD31
- Transmigration through the transmission Extravasation and migration into interstitial tissue
transmigrates into the tissue, the chemokines then give WBC a chemical trail to follow chemotaxis (movement towards the site of injury or infection is along a chemical gradient)
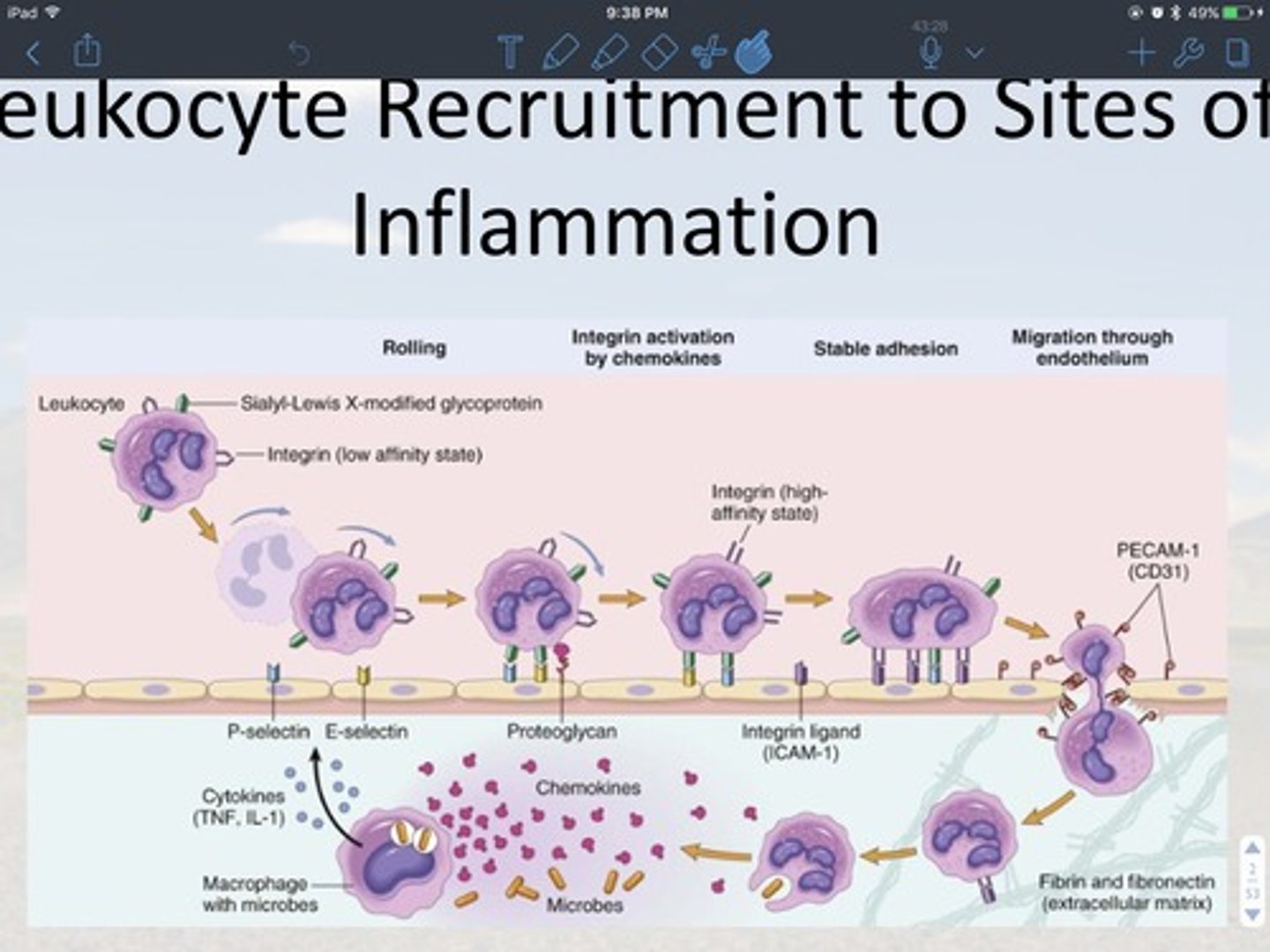
Describe phagocytosis
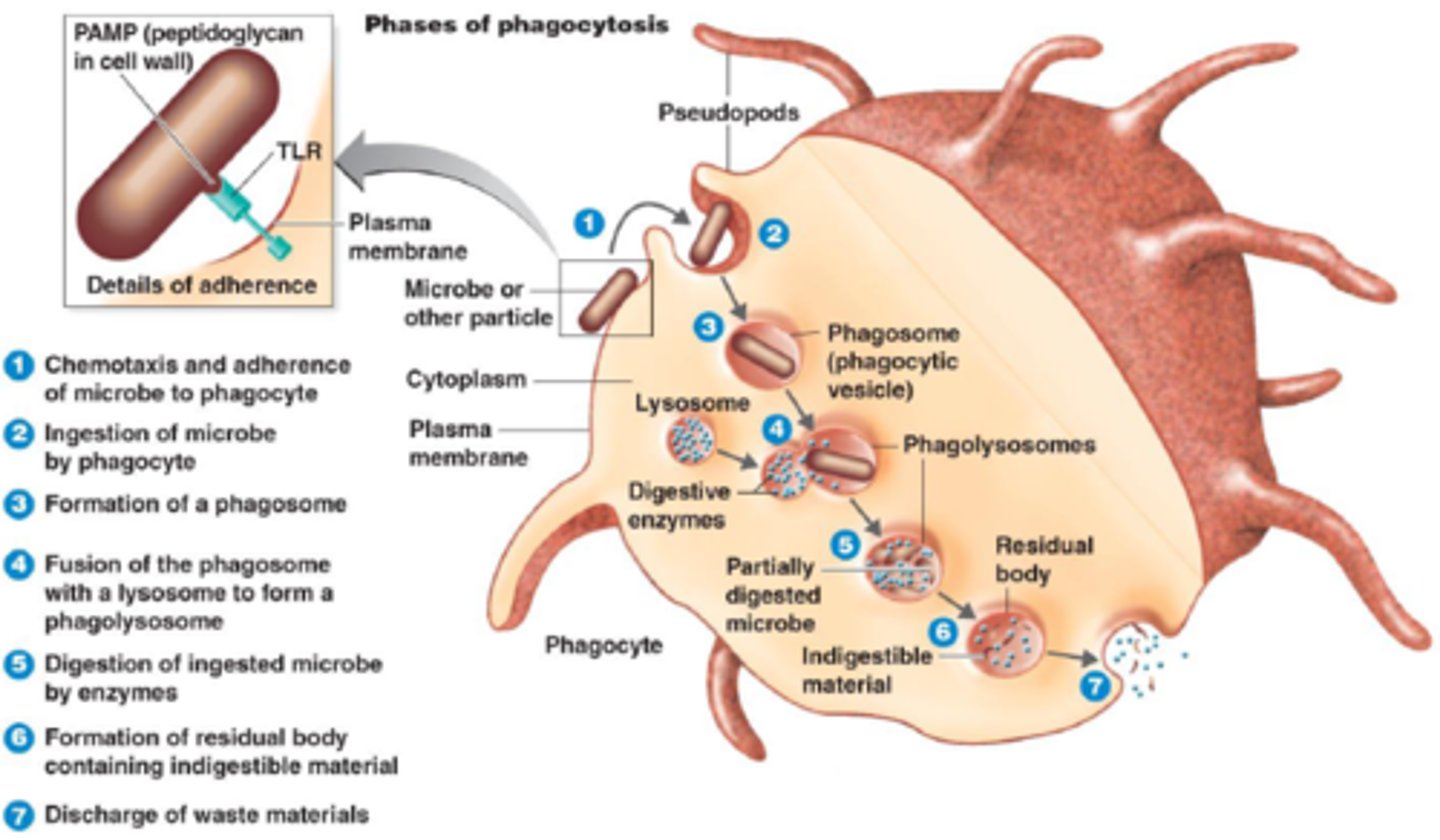
• Describe how a cell deals with ingested material
left in the digestive vacuole, which may then leave the cell by exocytosis.
• Know the function and source of
- Vasoactive amines
Two types: Histamine, serotonin
Histamine:
- Source: Stored in the granules of Basophil, platelets, mast cells near vessels
- Action: Vasodilation and vascular permeability
What is serotonin source and action
- Source: platelet granules, released during aggregation (when an injury occurs and the platelets stick together)
- Action: Vasoconstriction
- It is also neurotransmitter and regulates your intestines
• Know the function and source of: Complement
The complement system can be activated through three major pathways: classical, lectin, and alternative
3 entrance pathways that lead to a critical step: Cleavage of C3
- alternative (set off by microbes)
- classical (set off by antibodies)
- lectin (set off by mannose binding lectin)
what are the 4 main function in inflammation from the complement pathway
- C3a and C5A (anaphlatoxins) cause mast cells to release histamine which cause vasodilation and vascular permeability
- C3b binds to cells, tagging for phagocytosis
- C3a (and down stream C4a and C5a) recruit and activate WBCs
- C5b-C9: forms MACs (membrane attack complex) and lyses microbes : just kill the microbe directly by forming a hole in the membrane
• Know the function and source of:
Kinin system
HMW kininogen to bradykinin which causes increased pain, vascular permeability, vasodilation, and smooth muscle contraction. And kallikrein which is chemotactic and activates Factor XII
draw diagram
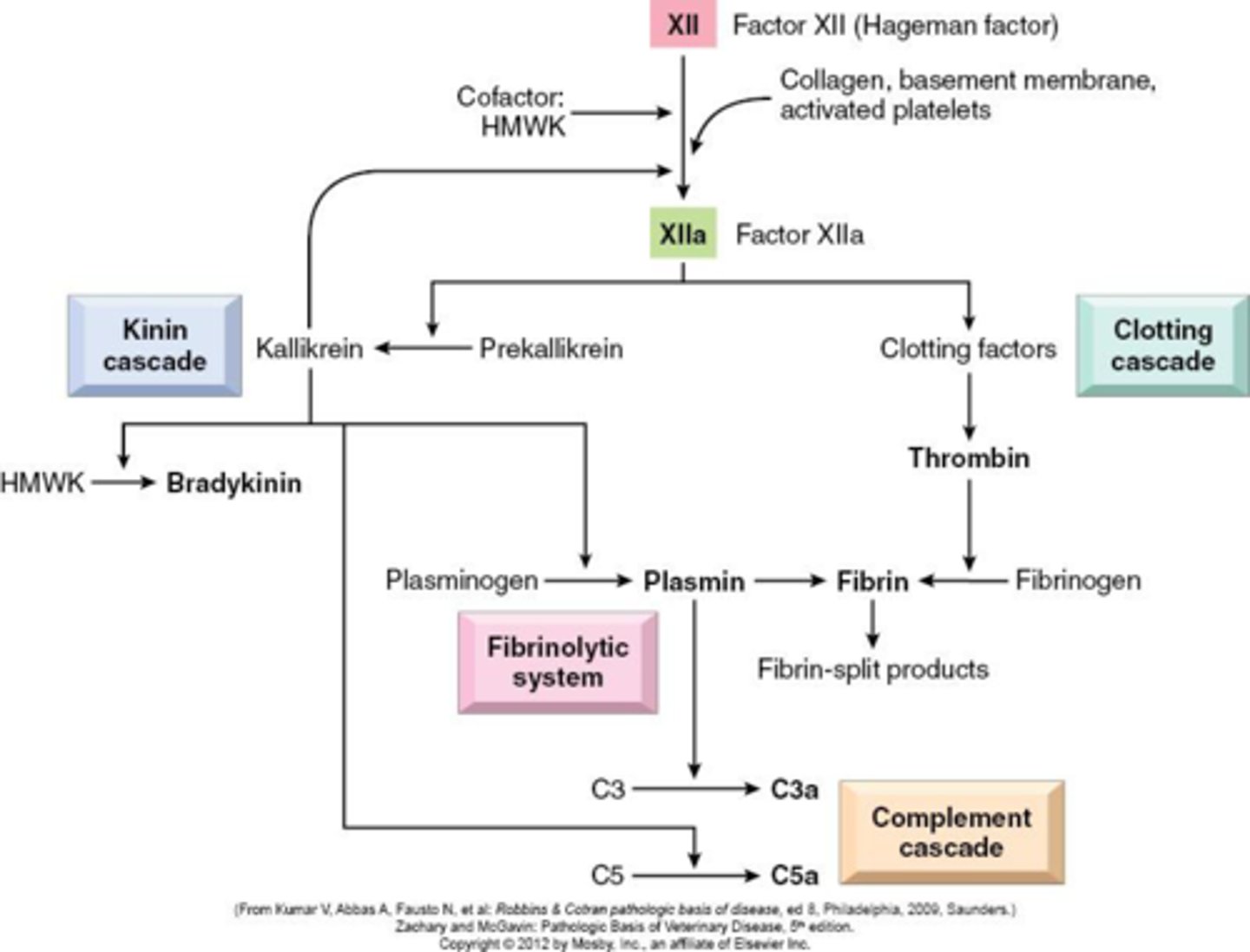
Know the function and source of: Coag system
- souce: hageman factor is key here because without it cascade can't start
- remains inactive until it encounters collagen or activated platelets which then set off a cascade that includes the coagulation, the kinin system, the fibrinolytic system (balance to coag) and complement
Know the function and source of: Arachidonic acid metabolism
A molecule that is made when cell membrane phospholipids are cut. So the fatty acid chain phospholipases cut the membrane which release AA
Activated by:
Chemical, physical, and mechanical trauma (cell injury) as well as other mediators (C5a)
Can happen anywhere you have INFLAMMATION in the body
classes: (these all balance each other)
- prostaglandins
- thromboxanes
- prostacyclin (**)
- leukotrienes
Know the function and source of: Acute phase proteins
- elevated levels of certain plasma proteins from liver
- most important: CRP, Fibrinogen, SAA
- stimulated by IL-6
Roles of APPs: act as opsonins and fix complement
Lab test:
- ESR (Fibrinogen)
- CRP
• Describe the inflammatory response from start to finish including wound healing
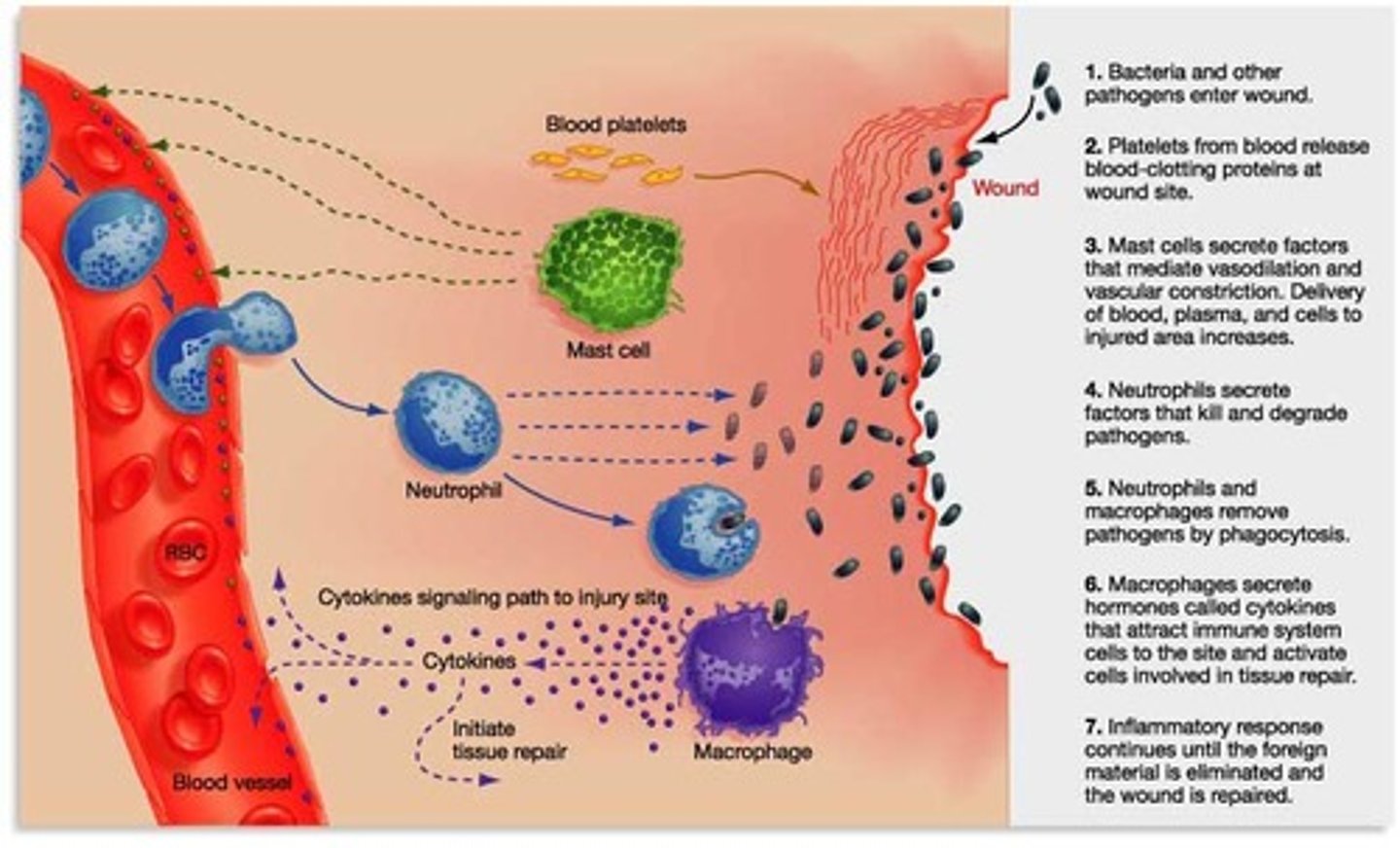
• Describe what happens when the inflammatory process cannot be completed
damaged tissues will not be able to repair
• Describe the systemic effects of inflammation
- Fever:
- pyrogens increase COX production to stimulate prostaglandin production in the hypothalamus
- Exogenous (LPS) or endogenous (IL-1, TNF) pyrogens
Resets the temperature at higher level
- raised heart rate and BP
- decreased sweating
- anorexia
- chills
- malaise
- sleepiness
• Describe the three types of cell populations. You should probably be able to give an example of each as well.
- labile: divide regularly (tissue: epithelium, blood and stem cells)
- stable: divide in response to need/ injury (liver, endocrine, kidney, bones)
- permanent: do not divide (nerve, hear, lens, auditory)
• What are the possible outcomes when healing takes place?
- regeneration
- scarring
What is the role of Growth factors in wound healing?
- proteins that stimulate cells proliferate, migrate, and differentiate
- function by binding specific receptors that send signal to the cell
- produced transiently in response to stimulus
What is the role of Fibroblasts in wound healing?
must be recruited to the area and proliferate. then they release proteins that build the ECM
- breaking down the fibrin clot, creating new extra cellular matrix (ECM) and collagen structures to support the other cells associated with effective wound healing
What is the role of Matrix metalloproteinases in wound healing?
Used in maturation and remodeling:
- MMPs degrade ECM in tightly controlled fashion to help manage any disorganized ecm production
What is the role of Extracellular matrix in wound healing?
- support
- place for adhesion and migration
- control of proliferation
two types:
+ interstitial (between tissue)
+ basement mem
three components:
+ fibrous
+ gels
+ glycoproteins
Describe the differences between healing by first intention and second intention
First Intention: clean edges, "slice" wound
1st: fibrin clot
2nd: neutrophils come in, increased mitosis in basement membrane cells
3rd: epithelials migrate and multiply in dermis, laying down basement membrane
4th: day 3: more macrophages the neutrophils. GRANULATION tissue starts, collagen production starts, epithelial layer thickens
5th: day 5: new vessels reach their peak, whole space is filled with granulation tissue, epidermis becomes mature and normal in thickness and architecture
6th: more collagen, more fibroblasts, inflammatory responses subside, more collagen, less vascularization
7th: end of 30 days: scar part is connective tissue, epidermis is normal, inflammation is mostly over
2nd: wounds involving extensive tissue loss, "scoop"
1st: BIGGER CLOT
2nd: MORE INFLAM
3rd: MORE GRANULATION TISSUE = LARGER SCAR
4th: WOUND CONTRACTION
What factors can interfere with healing?
- infection
- Nutrition
- Steroid treatment
- mechanical problems
- foreign bodies
- extent
- Location
- problems with cells and ECM deposition
- Others: age, diabetes genetics
Which cells are professional APCs and what do they do?
Dendritic cells: capture and present antigens to Tc. Express MHC class II and T cell costimulatory molecules. Found in the Tc zones in lymphoid tissue
Macrophages: phagocytize and present antigen. After phagocytosis that, macrophages load the chopped up bits into MHC class II molecules
B cells:
RECOGNIZE and PRESENT antigen
1. Recognize antigen via BCR
2. Take it in process it
3. Present via MHC class II molecules to helpers Ts
APCs capture and display the Ag "flag" in MHC class II molecules
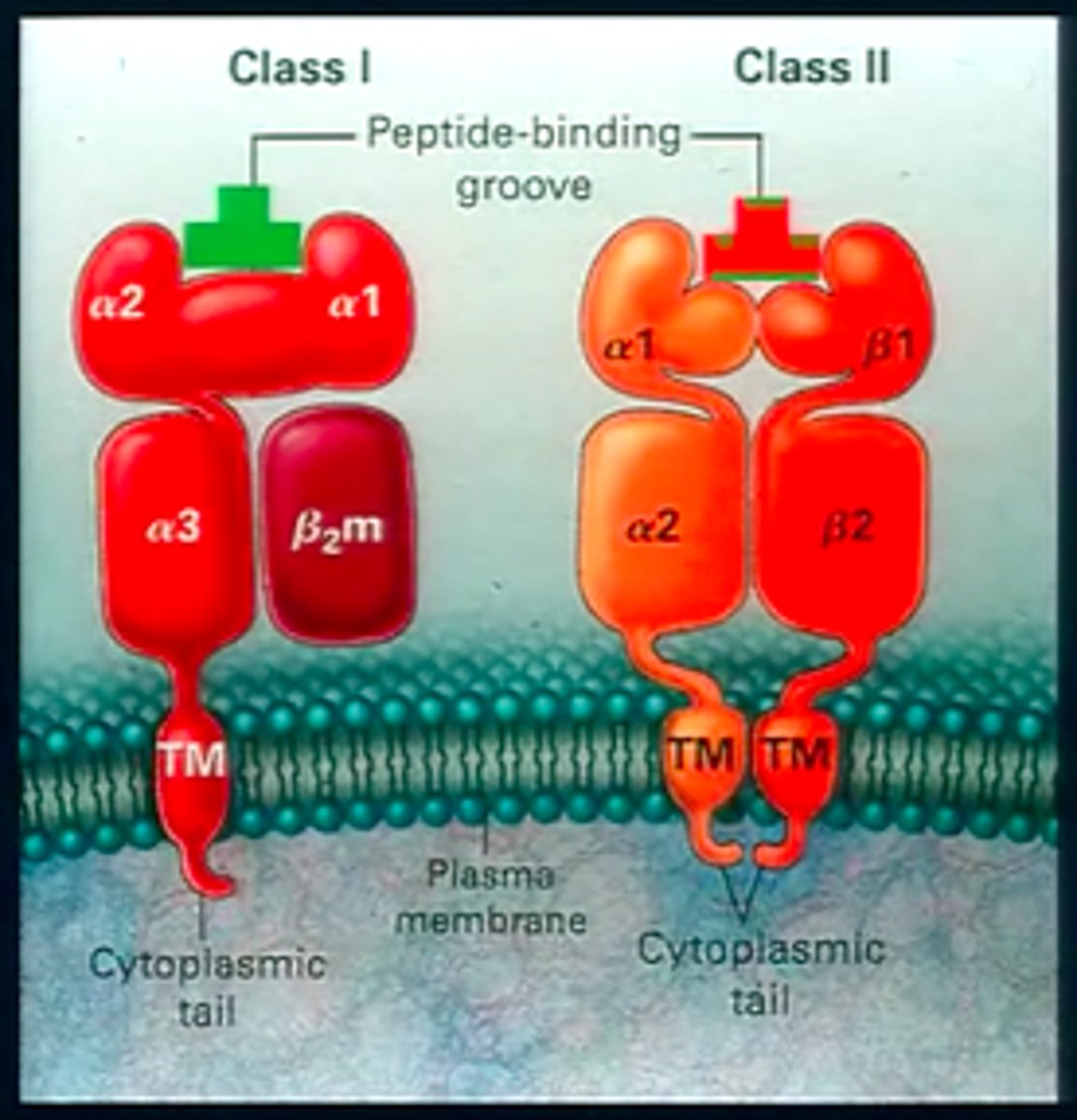
Tell me about the two classes of MHC molecules: where are they found, what do they do, and what do they look like
MHC class 1:
- where are they found: present on all nucleated cells
- what do they do: signal to CD8+ cytotoxic Ts (direct killing cells).
- what do they look like: Heavy alpha and beta 2 microglobulin chain
MHC class 2:
- where are they found: present on professional APCs (this cells only job is to present antigen to t cells).
- what do they do: Antigen is consumed, processed and presented in MHC II by these cells.
- what do they look like: Two peptide chains of more equal size. Signal recognized by CD4+ helper Ts
List and describe the five classes of antibodies
IgG:
- 70-80% of plasma
- monomeric
- diffuses out of vasculature
- neutralizes toxins and opsonize bacteria
- only class that crosses placenta
IgM:
- Largest, a pentamer
- units joined together at Fc fragments by J chain
- 10 binding sites, good for repeating epitopes
- first after immunization
IgA:
- Mucosa, tears, sweat, bile, breast milk
- Dimer, J chain connection
- stops bacteria and viruses from entering the epithelium
IgE:
- Fc fragment binds to receptors on mast cells
- Ag-binding results in release of granules (histamine, leukotrienes) and calls eosinophils
- parasite infections
- involved in hypersensitivity
IgD:
- present on some B cells
- Helps with antigen recognition/activation along with IgM
- Secreted IgD helps "arm" some basophils and mast cells in mucosa to help with immune surveillance
Describe the functions of the following cells and how they interact if they do: B cells
bone marrow derived
- Act in humoral immunity, producing antibodies (plasma cells)
- found in bone marrow circulation, spleen, MALT, lymph node follicles
- they recognize antigen in many forms, don't require MHC presentation
B cells receptors:
- BCRs have unique antigen specificity
IgM = part of BCR plus alpha and beta proteins
- Diversity of the Ig prevent on different B cells is result of gene rearrangement in V(D)J genes as in TCRs
differentiated B cell function (B cell that present antigen to Thelper):
Plasma cells make antibody:
- antibodies have the same specificity as that original BCR
- Affinity maturation or fine-tuning of antigen binding occurs
Memory cells:
- long-lived and ready to respond
- anamnestic response
Describe the functions of the following cells and how they interact if they do: T helper cells
thymus derived:
- Helper (CD4+) cells help humoral immunity:
- release cytokines which help stimulate B cells to make antibodies and encourage macrophages to destroy microbes
- different classes of helper T cells play different roles
- Th is usually the abbreviation used
HAVE TO BE PRESENTED ANTIGEN
Every Tcell has a TCR (T cell receptor)
- membrane bound
- two peptide chains, that together form a binding site that recognizes a unique antigen
- the variability is created by rearrangements of the gene segments called V,D,J
Describe the functions of the following cells and how they interact if they do: Cytotoxic lymphocytes
Cell mediated immunity (CD8+)
- involved in direct killing of cells (similar to Natural killer cells but the signal is different)
- cytotoxic Ts (CTL, Tc)
- cytokine production ( Releases perforins and granzymes, triggers caspase cascade)
DRAW DIAGRAM
Tell me how a humoral response develops during: T cell dependent B cell activation
DRAW DIAGRAM
T-cell dependent: most antigens aren't able to activate B cells
- they need T helper (CB4+) lymphocytes to develop a response
1. BCR recognizes antigen
2. antigen is ingested and chopped up
3. pieces are displayed on MHC class II on surface
THEN
4. CD4+ T helper cells recognize the presented antigen
Tell me how a humoral response develops during: T cell independent B cell activation
T-cell independent: doesn't require T cell help, EMERGENCY response. This occurs when the B cell has multiple copies of the antigen are present close together and engage multiple BCRs on a cell surface
- stimulates proliferation of B cell line, differentiation into plasma cells and antibody production
- antibodies are IgM
- doesn't result in memory
Know what these cytokines do in adaptive immunity: TNF
fever, inflammation, muscle wasting
- innate immunity and inflammation
Know what these cytokines do in adaptive immunity: IL-1
- Pro-inflammatory
- sources: Macrophages, B cells, DCs
Functions:
- Pyrogenic (produce fever)
- pro-inflammatory
- proliferation and differentiation
- BM cell proliferation
- innate immunity and inflammation
Know what these cytokines do in adaptive immunity: IL-2
- Adaptive immunity
- source: Th1 cells
*** clonal expansion******
function:
- Proliferation of: TCELLs
- B cells
- activated T cells
- NK cell function
Know what these cytokines do in adaptive immunity: IL-3
- Adaptive immunity
- source: T cells
function:
- Hematopoietic precursor proliferation and differentiation
- hematopoiesis
Know what these cytokines do in adaptive immunity: IL-4
- Adaptive immunity
- source: Th Cells
*******B cell differentiation into plasma cells**********
function:
- Proliferation of B and cytotoxic T cells
- enhances MHC class II expression
- stimulates IgG and IgE production
Know what these cytokines do in adaptive immunity: IL-6
- Pro-inflammatory
- source: Th Cells, macrophages, fibroblasts
**********cytokines is an important driver of the acute phase response********
function:
- B-cell differentiation
- Adaptive immunity
Know what these cytokines do in adaptive immunity: GM-CSF
- Adaptive immunity
- source: T cells, macrophages, fibroblasts
****cytokine that recruits more white blood cells from the bone marrow*****
function:
- Growth and differentiation of monocytes, and eosinophil, granulocytes production
- hematopoiesis
Know what these cytokines do in adaptive immunity: M-CSF
- Adaptive immunity
- source: Fibroblasts, endothelium
function:
- Monocyte production and activation
- hematopoiesis
Know what these cytokines do in adaptive immunity: G-CSF
- Pro-inflammatory
- source: Fibroblasts, endothelium
function:
Granulocyte production
- hematopoiesis
What is class switching in immunoglobulins and how does it happen?
- Constant region of the heavy chain is changed to another type (Fc)
-ANTIGEN SPECIFIC
-switch to IgG (TH1), IgE (TH2), or IgA is dependent on cytokines signals from T helpers
++only happens in t cell dependent b-cell activation
What are the four types of hypersensitivity? Describe each one.
Type I:
- Various names: immediate, Allergy, reagin-activated
(Hay fever, reaction to medication)
- Cells involved: Th2, IgE and mast cells are central to this response
Type II:
Names: cytotoxic hypersensitivity or antibody mediated
- antibody against some tissue component epitope is key here
Type III:
- Immune-complex mediated
- antigen/antibody complexes are the main problem
- size of the complexes influences outcomes
- amount of antigen and antibody matters too
Type IV: cell mediated/ Delayed (only one that doesn't have to do with antibodies)
- sensitized T cells do the damage
A; ALLERGY : Type I
C; CYTOTOXIC antibody-mediated: Type II
I; IMMUNE COMPLEXES: Type III
D; DELAYED: Type IV
Explain the Type I Hypersensitivity Reaction and what do you see
1. exposure to antigen/allergen:
- Th2 helper cells are activated and influence class switching to IgE production
- Lots of IgE is produced if persons susceptible
- Binds to mast cells via the Fc portion = sensitized
2. exposure to antigen/allergen:
- Mast cells release mediators such as: histamine, proteases, prostaglandins, and leukotrienes from granules and synthesize others from lipids
- they also secrete cytokines (late phase)
See:
- hay fever symptoms
- asthma
- conjunctivitis
- dermatitis
- hives
- GI upset
- anaphylaxis
Explain the Type II Hypersensitivity Reaction and what do you see
1. Antibody binds to epitope: three outcomes that lead to destruction
1. phagocytosis (opsonization)
2. Complement activation
3. Antibody dependent dysfunction (activate receptor)
what we see: depend on the antibody specificity
some examples:
Immune hemolysis:
- hemolytic disease of the newborn due to Rh
Drug-induced:
- drug acts as carrier for a hapten
- drug unmasked something that antibody could bind
Grave's disease:
- TSH receptor gets activated when it shouldn't
Explain the Type III Hypersensitivity Reaction and what do you see
Most of the time, when AB/AG complexes form, it is no big deal
- unless, there are lots of them and they get deposited in the tissue:
larger complexes usually get cleared pretty well, lots of antibody and the deposits it is usually local, lots of antigen and the depositing is more widespread bc the smaller complexes are more soluble
- once deposition happens, that causes inflammation: complement activation, vasoactive amines, platelet aggregation
out comes:
- vascular smooth muscle damage: necrosis
- Example:
- glomerulonephritis if deposition is there
- arthritis is deposition into joints
- serum sickness (rare) large amounts of foreign proteins for passive immunization
- specific antibody formed, complexes with circulating antigen and is deposited in various tissue
Explain the Type IV Hypersensitivity Reaction and what do you see
Delayed-Type hypersensitivity
- Ex: RXN in tuberculosis test, allergy tests
- happens 12-48 hours after exposure
- T effector cells secrete cytokines
- prolonged response becomes granulomatous inflammation
Mechanism:
- cytotoxic lymphocytes:
kill cells showing the antigen
rejection of transplants occurs through this mechanism
out come:
- Inflammation
- granulomatous Inflammation
- tissue/organ rejection
What do antibodies do?
Bind to antigens on pathogens to disable and clump them together ready for phagocytosis
Functions:
- Neutralize bacteria and their toxins
- opsonization
- ADCC
- Activate complement:
Lysis and more opsonization, Inflammation
How does autoimmunity develop?
the immune system makes a mistake and attacks the body's own tissues or organs.
four general mechanisms for the loss of self-tolerance and the development of autoimmunity:
1. previously secluded tissue proteins are exposed
2. Alteration of tissue antigens: Injury, infection, environmental insults, drugs
3. exposure to 'shared' determinants exogenous antigens similar to self antigens
4. impaired T cell regulation
Diseases to understand: HIV/AIDS
also known as Acquired immune deficiency syndrome:
- Viral Infections: HIV (HIV1, HIV2) spreads through contact with blood and bodily fluids
- Two major targets:
immune system and CNS
- Mechanism:
Depletion of CD4+ lymphocytes (T helper cells) -> serious immunosuppression -> opportunistic infections, neoplasms, neurologic problems
- Effects in immune sys:
- CD4+ T cell loss
- Defective DC and Macrophage function
- damage to lymphoid tissue (LATE)
- Effects in central nervous sys:
- monocyte/macrophages lineage (also have CD4) microglial cells infected
- probably gets there in infected monocyte
- neurons not infected
- actual damage not well understood
HIVs tropism:
- cells that express CD4
- Th Cells, macrophages, and dendritic cells gone
Diseases to understand: SLE
Autoantibodies: classic are ANA antinuclear antibodies
immune complex: deposition causes organ damage (esp. skin, joints, kidney)
most common in females more specifically black middle aged women
genetic predisposition: HLA DR2, DR3
Most common symptoms:
- youngish female, butterfly rash of face, fever, pain and swelling in joints, chest pain, and photosensitivity
as immune complexes build up, damage can manifest as nephritis, skin lesions, arthritis
- there can also be hematologic and neurologic problems
- prone to infection
- may have flares and remission
- death usually caused by renal failure, heart problems or infections
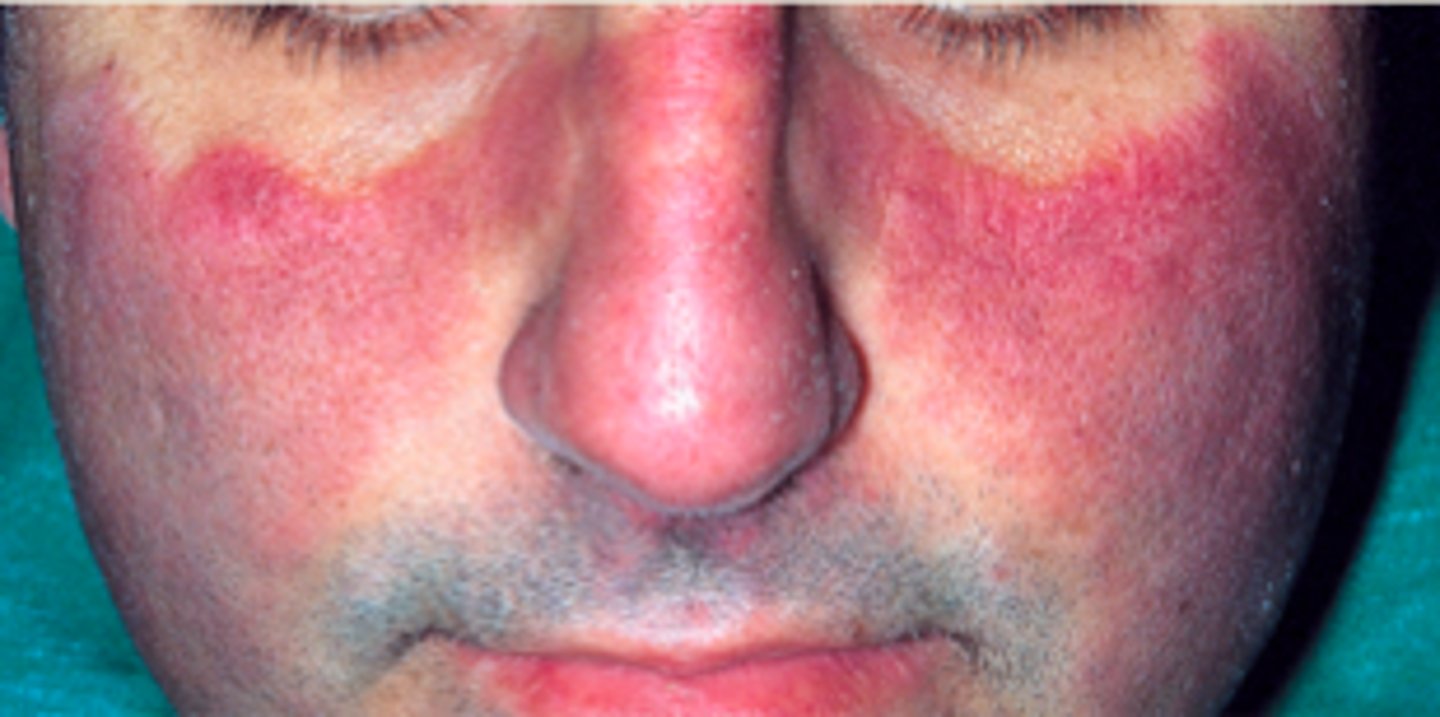
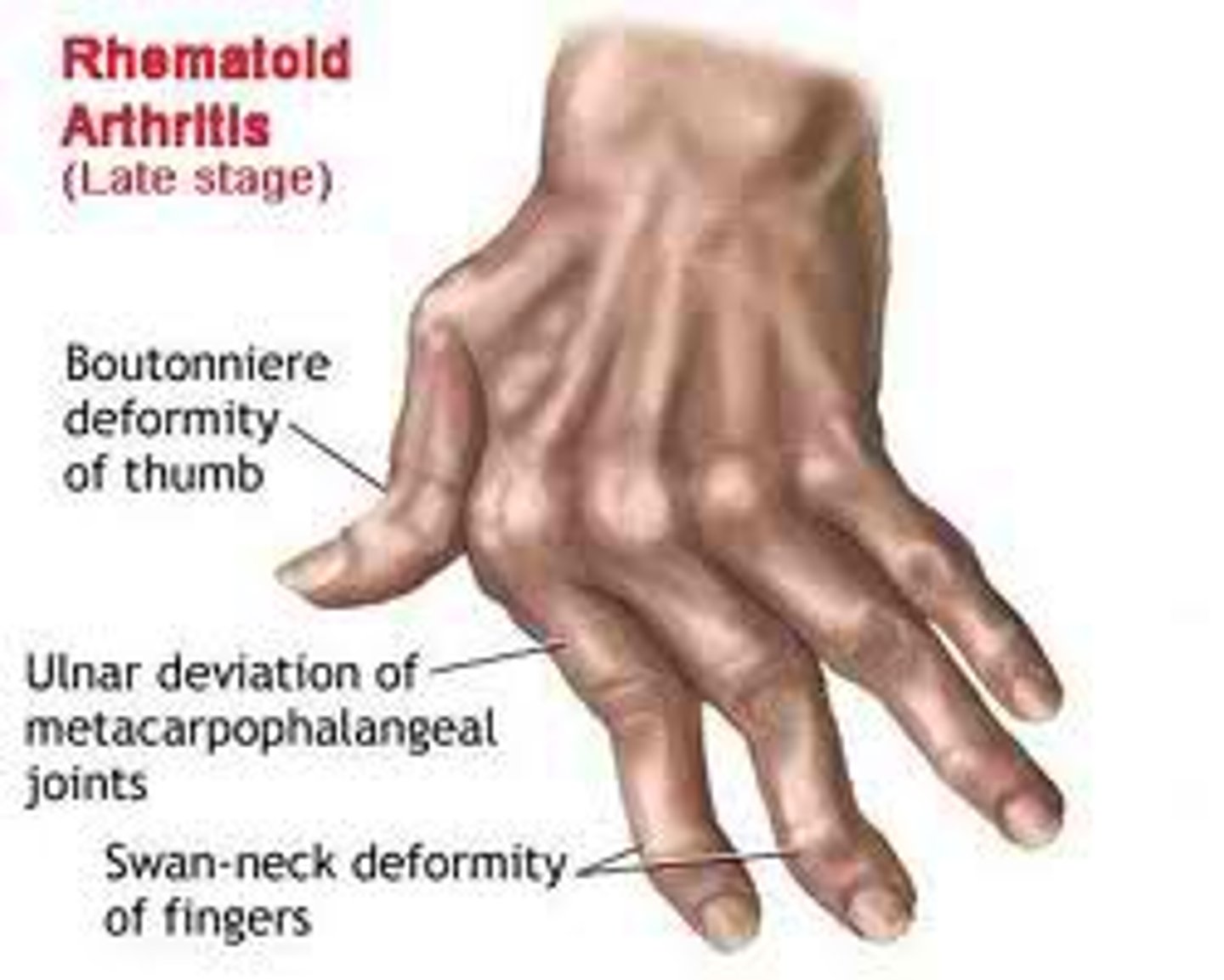
Diseases to understand: RA
Rheumatoid arthritis (RA)
mainly joint disease
- immune complex deposition and damage from cytokines
- 3-5x more likely in females, 20-40 years old
- genetic and environmental factors play a role
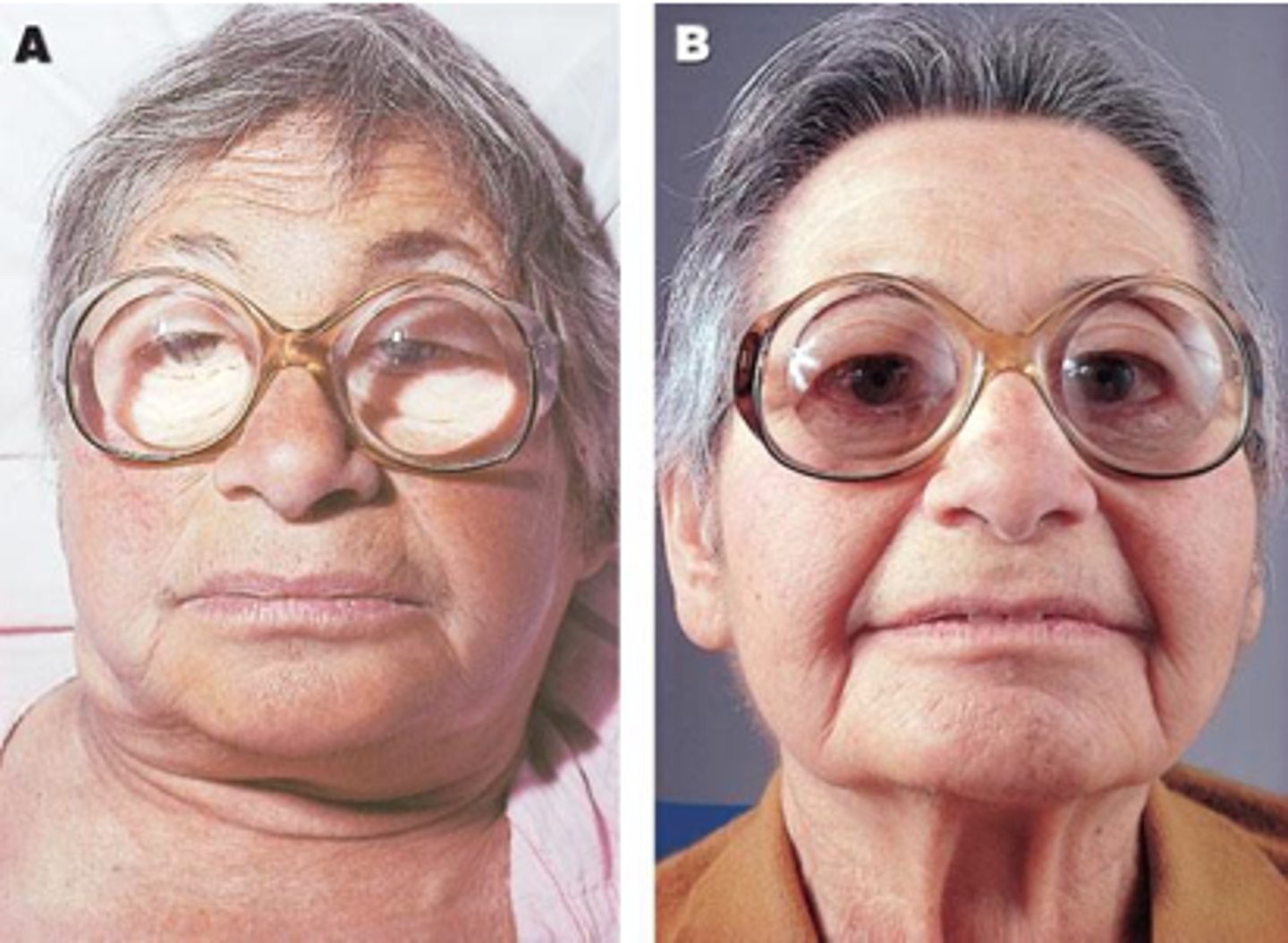
Diseases to understand: Hashimoto's
organ specific: thyroid
- autoantibodies to thyroid tissues lead to destruction of thyroid epithelial cells
- t cell mediated :
- CD8+ cell death
- ADCC
symptoms:
- enlarged thyroid
- hypothyroidism
- usually middle-aged females
- may have other autoimmune diseases as well
normally in middle aged women who are predisposed to genetic conditions
What's the difference between primary and secondary amyloidosis?
primary:
- deposition of amyloid w/o any concurrent or previous disease
- monoclonal plasma cell proliferation
- multiple myeloma
secondary:
- follow identifiable disease
- often chronic inflammation
- rheumatoid arthritis
What do all amyloid diseases have in common?
Extracellular proteins deposited as fibrils made of BETA - PLEATED SHEETS Very think sheets causes accumulation
What is the origin of the amyloids in Immune origin
primary: immune origin amyloidosis:
- AL amyloid light chain type (too much light chain being produced by plasma cells)
- protein precursor: Ig light chain
(intact light chain, Amino terminal of light chain, and usually light chain)
- Source: single B lineage clone
What is the origin of the amyloids in Reactive amyloidosis
Secondary: reactive systemic amyloidosis:
- protein precursor: serum amyloid A (SAA)
+ produced by the liver usually in small amounts
+ following inflammation it rises (1000 fold)
+ singlas for up regulation (IL-1,TNF-alpha, IL-6)
+ not the whole story, something else involved
• What does SAA have to do with inflammation and reactive amyloidosis?
SAA is an acute phase response protein. when the acute phase response occurs and inflammation happens it rises (1000 fold). IL-1,TNF-alpha upregulates IL-6 which tells the liver to make lots of SAA. Sometimes misfolds into a beta pleated sheet and vibrals and deposit.
It is the protein precursor for reactive amyloidosis
• Explain what MHC classes I and II (HLA) have to do with transplantation.
HLA human leukocyte antigen: Human version of MHC. They are cell surface proteins that are involved in presenting antigen. What we match during looking for a donor
Summary: host APC cell is behaving normally presenting antigen to the host t cells. The antigen that is loaded into the MHC or HLA is the DONOR ANTIGEN
• Explain the major types of transplant rejection events
Hyper acute:
- minutes to hours
- preformed antibodies in recipient's plasma bind to graft endothelium
- Fibrinoid necrosis, ischemia and thrombosis
Acute:
- early, 7-10 days
- late, 11days-6weeks
can be cellular or humoral immunity that causes rejection
+when cellular:
+ happens within first month
+ CD4+ and CD8+ cells in organ with edema mild hemorrhage
+ renal problems
+ distinguish from drug toxicity from cyclosporine
+ humoral:
+ antidonor antibodies
+ necrosis, neutrophils, complement, antibody, fibrin, and thrombosis
Chronic:
- months to years post transplant
- antibodies bind to HLA on endothelium
- Arterial symptoms: thickening
- Kidneys: glomerular damage too, rising creatinine
• Explain how anti-rejection drugs work
- Azathioprine ( T cell mediated)
- Cyclosporin A (Inhibits clonal CTL expansion)
- Tacrolimus (T Helper lymphokine reproduction)
- Rapamycin (IL-2 receptor interference)
- Biologics: monoclonal antibodies against T helpers, monoclonal antibodies or lymphokines with toxins that target activated T cells.
• List risk factors for developing atherosclerosis
- factor V-leiden mutation
- prothrombin mutation
- disorder of (antithrombin III, Protein C deficiency/resistance, and fibrinolytic pathway disorder)
- taking oral contraceptives
- related to estrogen concentration pills (increase in fibrinogen and Vita K dependent clotting factors. Decrease in antithrombin II and increased risk in pregnancy)
- malignancy
- nephrotic syndrome
- obesity, age, and smoking
Immune risk factors:
- HIT syndrome: autoantibodies bind heparin and platelet membrane
- antiphospholipid antibody syndrome (endothelial injury, platelet activation, interaction with coag factors)
• Explain how atherosclerosis develops
1st
• What are some clinical symptoms in patients with atherosclerotic plaques?
- unstable angina
- myocardial infarction
- cerebral infarction
- aortic aneurysm
- peripheral vascular
• Explain hemostasis. Include platelets and coag factors and what all has to happen to make a clot and limit the clot
hemostasis: the stopping of a flow of blood
- Vasoconstriction
+ Serotonin and ENDOTHELIN proteins
+ Transient effect (lasting only for a short time because we need vasodilation to happen later for the inflammatory process)
... which brings in platelets which ....
- Platelets: A A A
+ Damage to the endothelial layer exposes ECM
+ This facilitates platelets adherence, activation, and aggregation
+ this formation of the initial platelet plug is call primary hemostasis
- Coag Cascade (or secondary hemostasis is going to stabilize clot)-> Fibrin
+ tissue factor and Factor VII activate the coag cascade or factor XII or Hageman Factor activate
- Fibrin -> stable clot
+ thrombin cleaves fibrinogen which creates a fibrin clot
+ thrombin also activates platelets which reinforces plug
- Counter regulation stops process (Fibrinolysis)
+ TTPA gets activated: also known as Tissue Type Plasminogen as and activator cleaves plasminogen to plasmin which degrades fibrin
• List and describe the parts of Virchow' Triad
- changes in the intimal surface of vessel:
+ injury:
++anything that removes or changes the endothelium
+++ exposes the ECM beneath will activate platelets
+++ changes in the coagulation influence
++ Trauma, chemical injury
++ unstable atherosclerosis
+ other inflammation
++ heart valves in endocarditis
++ immune complex mediated inflammation
++ any blood vessel passing through inflamed area
- changes in the pattern of blood flow:
+ turbulence can:
++ cause endothelial injury, form counter current or stasis, promote endothelial dysfunction
+ turbulent blood flow for artery thrombosis
(branching points, atherosclerosis narrowed areas)
++ platelets tend to collect there
+ stasis contributes to:
++ platelets and WBCs contacting endothelium more than usual
++ slowing movement of pro and anti coagulative agents
LEADS (stasis in the heart): aneurysms, congestive cardiomyopathy, myocardial infarct, mitral valve problems
+ speed of the blood flow contributes to vein thrombosis due to stasis:
++ general: congestive heart failure decreases blood pressure
++ local: leg thrombosis due to sitting too long
- changes in the constituents of the blood:
+ more risk for venous thrombosis than arterial and intracardiac problems
+ primary or secondary hypercoagulative states are possible
+ what are risk factors for hypercoag: (more platelets, more fibrogen, decrease in anticoag, stasis, increase in viscosity, procoag compounds)