Lecture on Telomeres and Cancer
1/11
Earn XP
Description and Tags
These flashcards cover key concepts related to telomeres and their role in cancer biology, cellular aging, and therapeutic targets.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
What is cellular immortality in the context of cancer?
Cancer cells bypass normal cellular aging
avoid senescence and crisis
maintain the ability to divide indefinitely.
What is the difference between senescence and crisis?
Scenescence: permanent cell cycle arrest where cells remain metabolically active triggered by:
telomere shortening
DNA damage
oncogene activation
Crisis is a phase marked by extensive DNA damage and widespread cell death due to critically short telomeres
from which a few cells may escape by reactivating telomerase and undergoing unregulated division.
What is the Hayflick Limit?
Normal human cells divide approximately 40-60 times due to telomere shortening, after which they enter senescence.
What are telomeres and their function?
Telomeres are repetitive DNA sequences (TTAGGG) at chromosome ends
protect chromosomes from degradation and shorten with each division due to the end-replication problem.
Describe the structure of telomeres.
Telomeres have a single-stranded 3' overhang that protects DNA
a Shelterin Complex of proteins that protect telomeres
T-loop formation where the overhang loops back to shield chromosome ends.
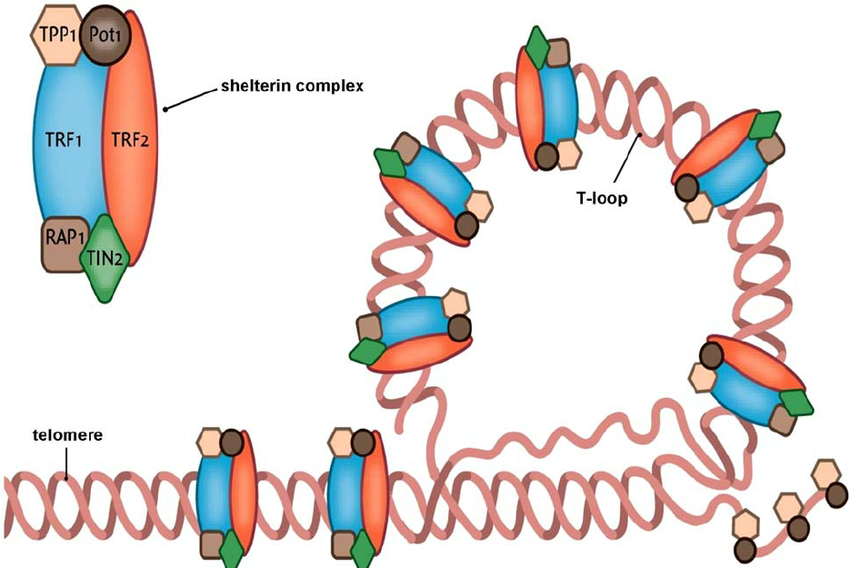
What happens during telomere shortening and crisis?
critically short telomeres trigger senescence or crisis
causes genome instability and cell death unless telomerase is reactivated.
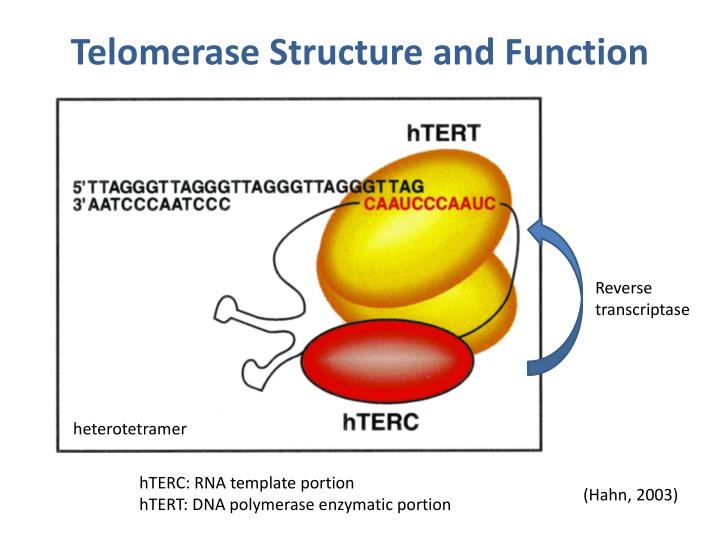
What is telomerase and how does it work?
Telomerase is a ribonucleoprotein enzyme complex that adds TTAGGG repeats to telomeres,
composed of:
hTERT (catalytic protein)
hTR (RNA template for repeat addition).
How do cancer cells use telomerase to become immortal?
Cancer cells re-express telomerase to maintain telomere length, preventing senescence and crisis, while normal somatic cells do not express telomerase.
Why is telomerase a promising target in cancer therapy?
Telomerase is active in 90% of cancers but inactive in most normal cells, and its inhibitors can induce crisis and kill cancer cells.
What is the role of Myc in telomerase activation?
Myc is an oncogene that upregulates telomerase expression, commonly found in Myc-driven cancers.
What is Imetelstat and its mechanism of action?
Imetelstat is a telomerase inhibitor that blocks telomerase activity, causing telomere shortening which leads to crisis and cancer cell death.
How is telomerase linked to pediatric tumors?
Some childhood cancers, such as neuroblastoma, depend on telomerase, with high telomerase activity linked to poor prognosis and worse survival outcomes.