BJU Physical Science, Chapter 7, 6th edition
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
chemical reaction
the process that changes a substance into one or more different substances
precipitate
A solid that forms from a solution during a chemical reaction.

example of a composition change
wood burns and leaves behind ash
chemical equation
a combination of chemical formulas and symbols that models a chemical reaction
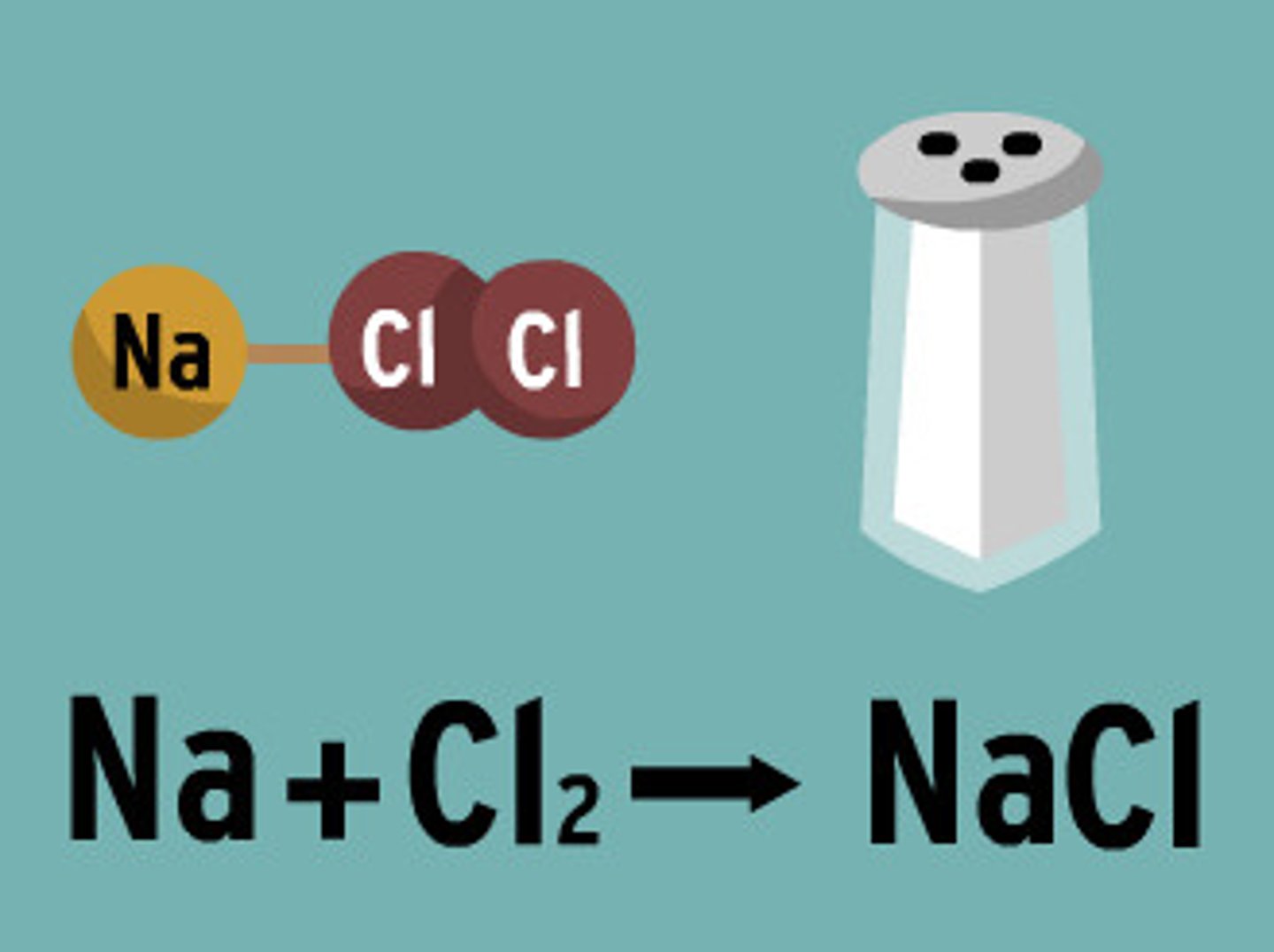
Reactants
elements or compounds that enter into a chemical reaction
Products
The elements or compounds produced by a chemical reaction.
arrow
yields
Coefficients
whole numbers that are placed in front of the formulas in an equation in order to balance it
synthesis reaction
a chemical reaction in which two or more substances react to yield a single product

decomposition reaction
a reaction in which a single compound breaks down to form two or more simpler substances

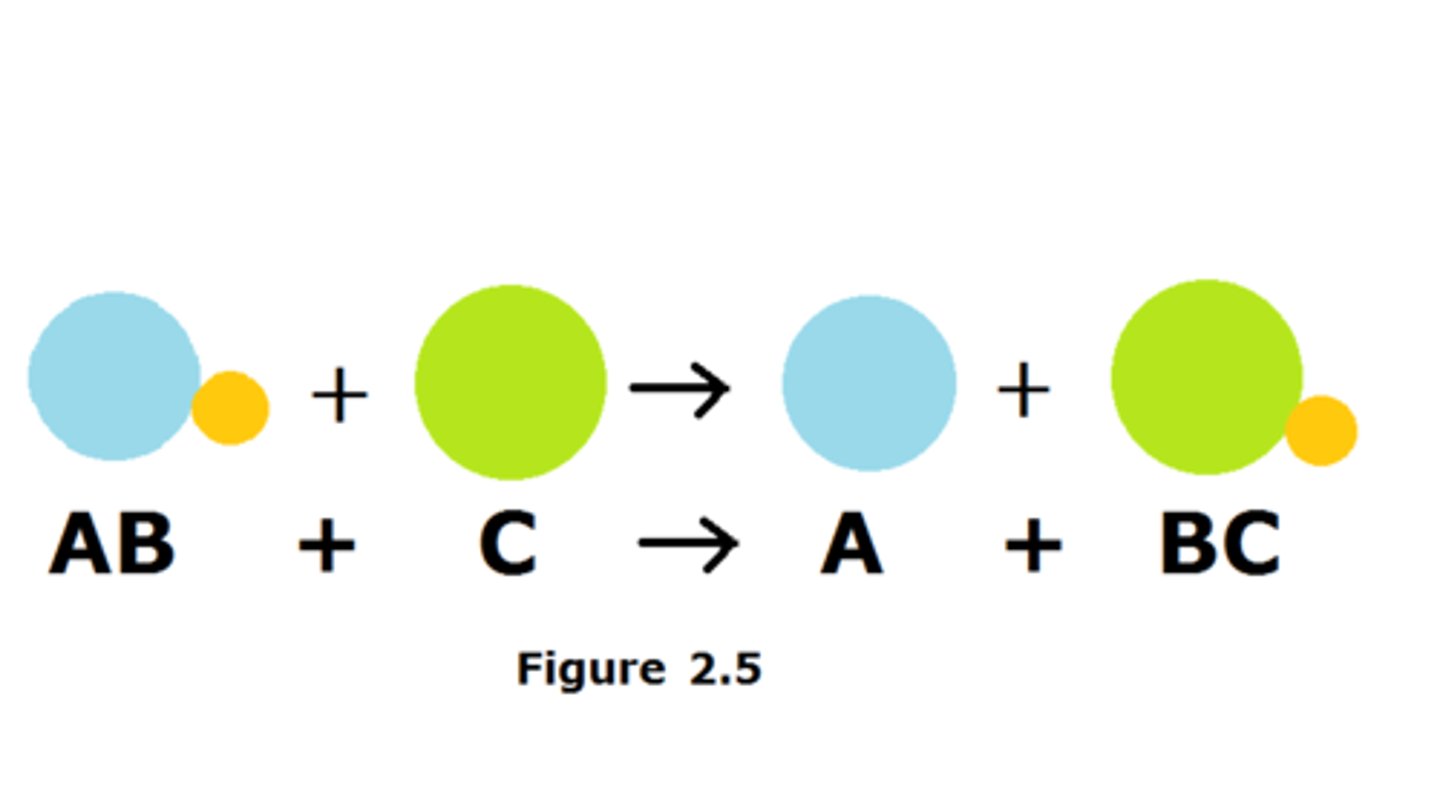
single replacement reaction
a chemical change in which one element replaces a second element in a compound
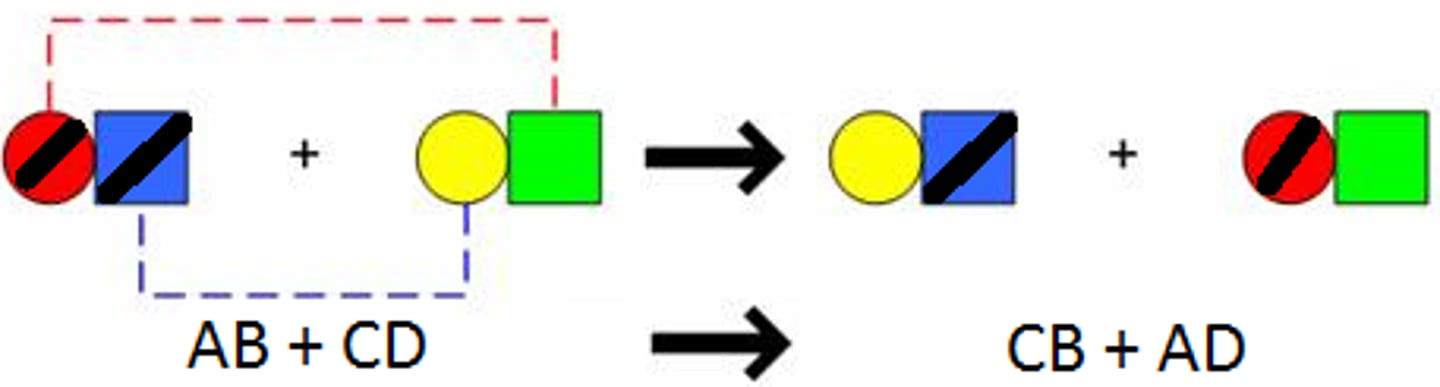
double replacement reaction
a chemical change that involves an exchange of positive ions between two compounds
combustion reaction
when a substance reacts with oxygen

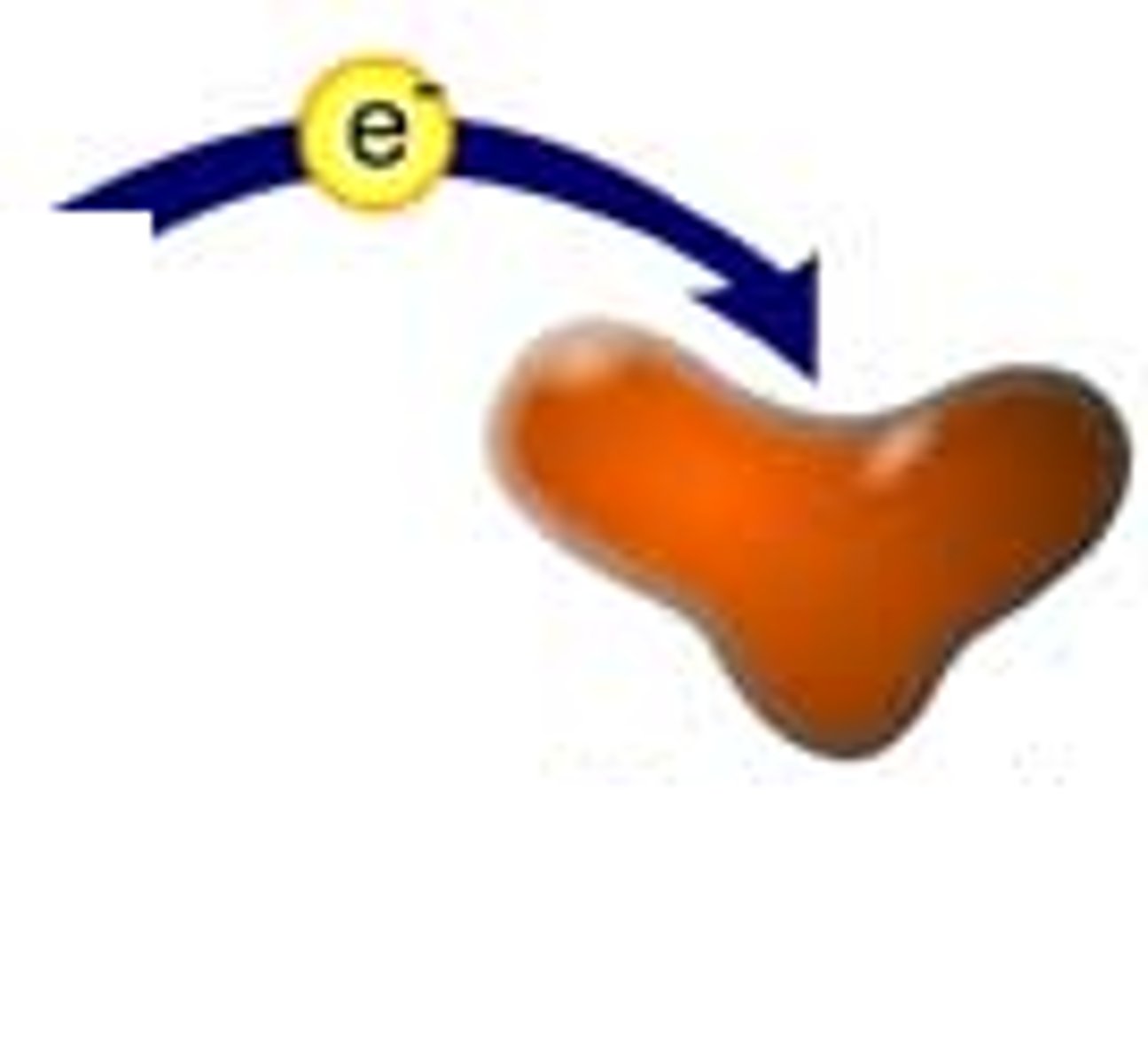
Oxidation
any instance in which electrons are lost
reduction
gaining of electrons during a chemical reaction
exothermic reaction
a chemical reaction in which heat is released to the surroundings

endothermic reaction
A reaction that absorbs energy in the form of heat

activation energy
Energy needed to get a reaction started
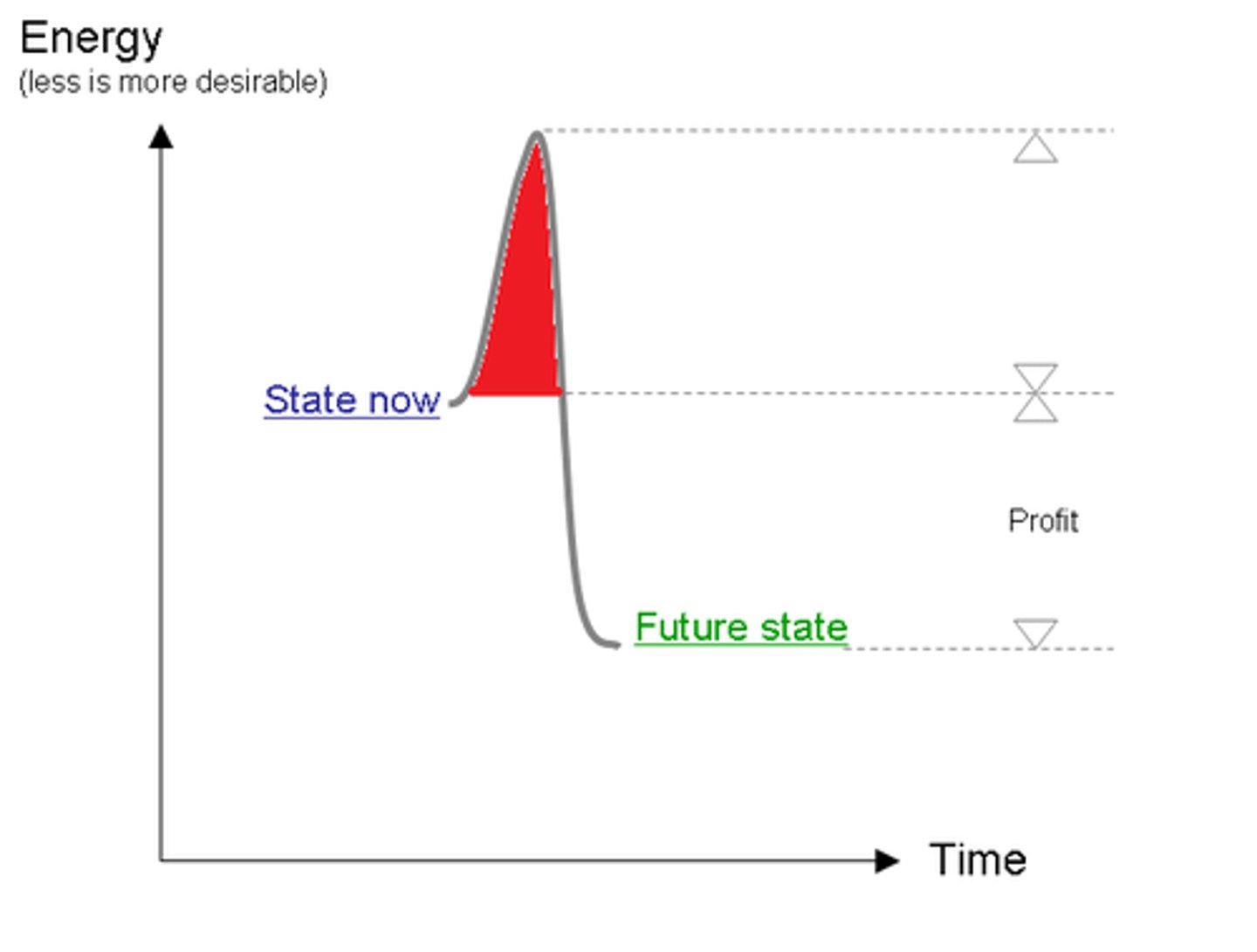
conservation of energy
Energy cannot be created or destroyed