EQ1- How does the carbon cycle operate to maintain planetary health?
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
The carbon cycle
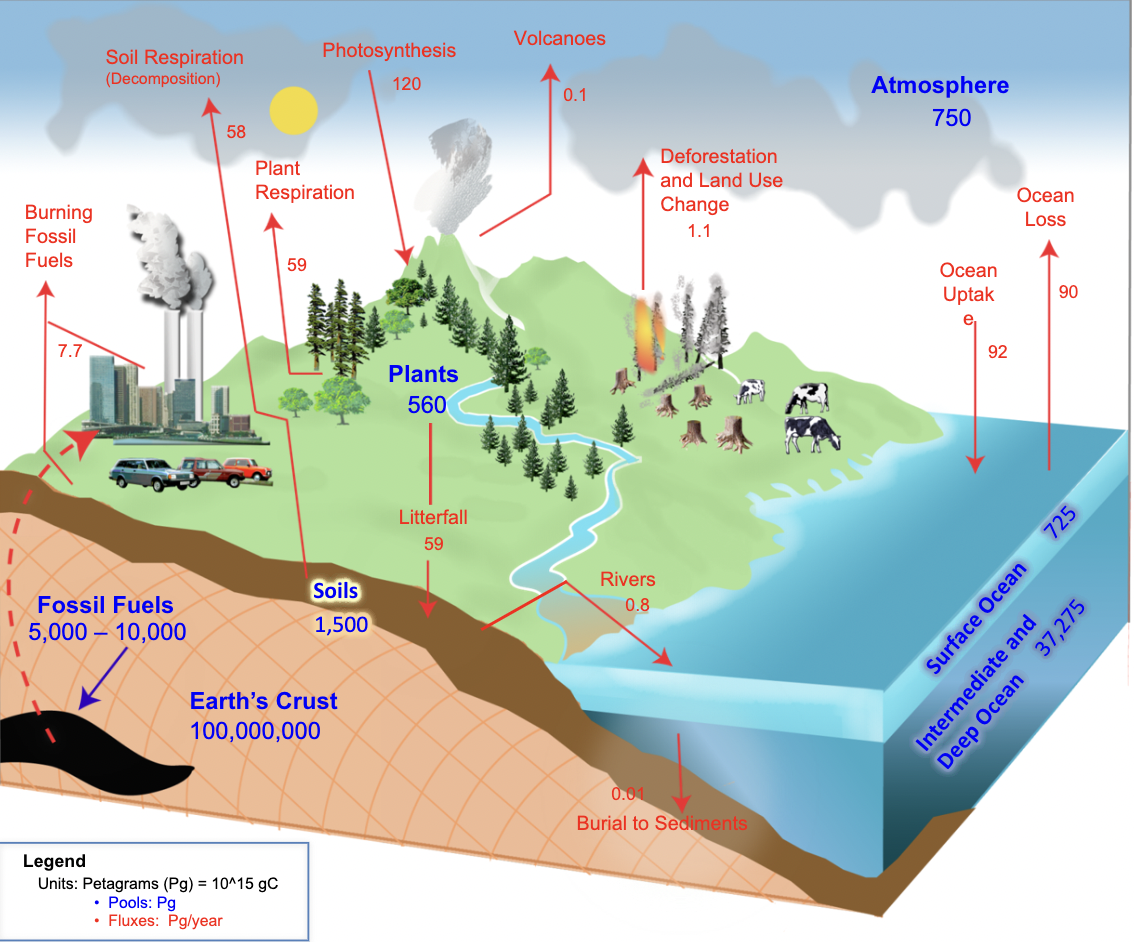
Cycle by which carbon moves from one Earth sphere (atmosphere- CO2 + methane, hydrosphere- dissolved CO2, lithosphere- limestone + fossil fuels, biosphere- living + dead organisms) to another.
It is a closed system, with no inputs or outputs, but components:
Stores: where carbon is held.
Fluxes(transfers): flow of carbon between different stores, measured in petagrams (PgC).
-these vary in size: the quickest cycle is completed in seconds during photosynthesis, whereas dead organic material takes years to be returned. Some organic material is buried in the sea + form sedimentary rock which is also extremely slow.
Processes: the physical mechanisms which drive the fluxes between stores e.g. photosynthesis, diffusion.
Biochemical carbon stores
Terrestrial
Carbon is stored in plants + animals. When they decay, it can be converted into other carbon stores by going into soil. Other terrestrial carbon is stored within mantle + can be converted to atmospheric carbon via volcanoes letting out CO2 during eruptions. Role of organisms:
Photosynthesis: plants remove CO2 from atmosphere using energy from sunlight to produce energy for plant growth.
Respiration: Plant/ animals release CO2 as they breathe and grow.
Decomposition: when living organisms die, they’re broken down, releasing CO2 into the soil.
Combustion: the burning of biomass/ fossil fuels by humans and in natural wildfires release CO2 back into the atmosphere.
Oceans
Aquatic plants+animals fall to the ocean bed after death. Due to compression + cementation, they form sedimentary rock. During these processes, crude oil + natural gas can form.
Atmosphere
CO2 is stored as a gas in the atmosphere. Carbon can leave the atmosphere when mixing with water vapour. When precipitation happens this falls as acid rain.
4 geological carbon stores
In formation of sedimentary rock, carbon can be found in several different forms:
Limestone
Crude oil
Coal
Natural gas
Formation of limestone
Marine organisms such as molluscs+coral have carbon-based shells formed from calcium carbonate.
When they die, they sink to the bottom of the ocean.
As more sediment falls on top of them, they’re compacted. When sediment reaches 100m in depth, the pressure+chemical reactions cause cementation to take place. This leads to formation of limestone.
Formation of crude oil
Biologically degraded material from dead organisms settle in ocean floor.
Anaerobic reactions occur with intense heat+pressure, turning majority of organic carbon into a liquid (crude oil).
Due to its light density, crude oil may migrate upwards through layers of permeable rock. But a layer of impermeable rock will result in crude oil being trapped.
Formation of coal
Land-based plants die + enter swamps, they slowly settle→ under heat+pressure as more sediment builds up on top, they compact to form peat and coal.
-coal takes millions of years to form, depending on temp + pressure.
Formation of natural gas e.g. methane
Methane is created as a by-product during the formation of crude oil.
Natural gas is trapped within the same sedimentary layers that crude oil are found in.
2 geological processes that release carbon
Chemical weathering of rocks
Volcanic out-gassing
Chemical weathering
The wearing away of rock by chemical reactions, causing material to dissolve through solution, hydrolysis + oxidation.
Slightly acidic rain forms from CO2 in the atmosphere being dissolved into rainwater.
When acidic rain hits carbon rich rock e.g. limestone, it can dissolve material + form calcium carbonate.
These dissolved materials are then transported down rivers + deposited into sea, forming sedimentary rock.
Volcanic out gassing
Deep sedimentary rock is melted under intense heat+pressure, forming magma.
During an eruption, magma rises, pressure changes, and CO2 previously dissolved, trapped, frozen, or absorbed in magma, is released as gas bubbles (outgassing).
occurs mainly along mid-ocean ridges, subduction zones + at magma hotspots.
release CO2 at a rate of 0.3Gt/year
What is carbon sequestration?
The movement of carbon into carbon stores which can lower the amount of CO2 in the atmosphere.
2 types of carbon sequestration
Oceanic + terrestrial
Oceanic sequestering
CO2 can enter the ocean via diffusion through 3 mechanisms:
Physical pump
Biological pump
Carbonate pump
Biological pump
Phytoplankton covert CO2 into organic matter via photosynthesis, transforming atmospheric carbon into biological
they are consumed by other organisms. All of these consumer organisms will respire, returning some of the carbon to the atmosphere in the process.
biological decomposers will consume dead matter + respire, returning it to atmosphere.
Thermohaline circulation
A global system of surface + deep ocean currents, which circulate carbon, driven by differences in temperature + salinity.
Warm surface waters are depleted of CO2 and nutrients through evaporation but become enriched again through the circulation of currents.
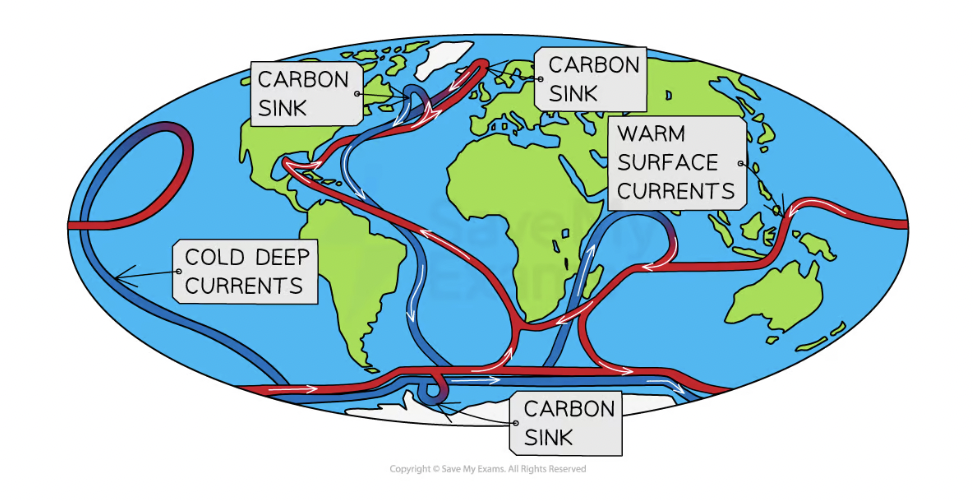
Physical carbon pump
CO2 absorbed by ocean surface via diffusion- this is taken from the surface down to the deep ocean stores through currents.
Thermohaline circulation distributes carbon around the planet.
Cold water absorbs more CO2 so as waters move towards the poles, more CO2 is absorbed. Salinity increases too, making the water denser + therefore water sinks taking CO2 down to deep ocean stores.
Through upwelling currents, previously stored carbon in the deep ocean stores returns to oceans surface as water warms + back into atmosphere.
Carbonate pump
When organisms with calcium carbonate shells die, they sink to the ocean floor. Here, these organisms can accumulate as sediment. Eventually this is transformed into sedimentary rock under pressure + compaction.
They can also be decomposed by bacteria, which can return carbon into the ocean in the form of dissolved organic carbon via thermohaline circulation.
Terrestrial sequestration
Plants (primary producers) sequester carbon out of the atmosphere during photosynthesis. Carbon enters the food chains + nutrient cycles in this way.
Respiration returns some of the carbon back into the atmosphere.
Consumers eat other organisms below them in the food chain. They then return the carbon from that organism through respiration.
Biological decomposers consumer dead organic matter + return carbon to atmosphere through respiration.
Biological carbon + where it is found
Dead organic matter found in the ground or soil contains biological carbon.
The biological carbon can be returned to the atmosphere when decomposed. This is dependent on temperature + climate.
Mangroves: Due to being submerged beneath tidal water twice a day, the soils are anaerobic (no oxygen). Biological decomposers can’t survive without oxygen so breakdown of material takes longer.
Tundra soils: Soil contains ancient carbon which is permanently frozen, stopping microbe activity that decays the material. This only happens once the surface layer thaws, making the tundra a massive carbon store.
Natural + enhanced greenhouse effect
Solar radiation enters atmosphere- most is absorbed by Earth’s surface whilst some is reflected back into space.
This radiation hits the ground which then gives off infrared radiation. This radiation is absorbed by GHG, stopping it from leaving the atmosphere.
GHG transmit heat back to Earth, allowing it to be at a high enough temp to support life.
Enhanced GH effect
Human activity causes there to be an increase in GHG in the atmosphere, so more radiation is retained in the Earth’s atmosphere than normal.
Burning of fossil fuels- release CO2 at rate of 10+Gt/year
Effect of GHG on temp + precipitation distribution
Temp: Different locations receive different levels of solar energy- the angle of the sun’s rays result in the equator receiving the most concentrated radiation, whilst at the poles the same radiation is dispersed over a greater distance.
Colour of the surface impacts how much radiation is absorbed (albedo effect)- white snow + ice caps reflect majority of heat whilst darker oceans + forests absorb heat. This heat is then redistributed via air circulation + ocean currents.
Precipitation: The heating of the Earth’s surface leads to warm air rising, cooling + condensing to form clouds. Intense solar radiation at the equator leads to warm air rising, causing high levels of rainfall all year.
Atmospheric regulation through photosynthesis
Relationship between soil health + carbon
Ocean + terrestrial plants photosynthesising keeps CO2 levels constant, helping the regulate average global temps. As a result, patterns in plant productivity + carbon density are evident.
High net primary productivity (organic matter available to consume) occurs in warm + wet regions e.g tropical rainforest or shallow ocean waters.
Soil health
Amount of carbon stored in soil depends on: inputs (plant litter + animal waste) and outputs (decomposition, erosion + uptake by plant growth).
Carbon within soil organic matter helps provide soil with its water retention capacity, its structure + fertility.
Fossil fuel combustion
Carbon in fossil fuels naturally flow into atmosphere very slowly through volcanic activity. However the burning of fossil fuels has increased the flow from slow to fast carbon cycling.
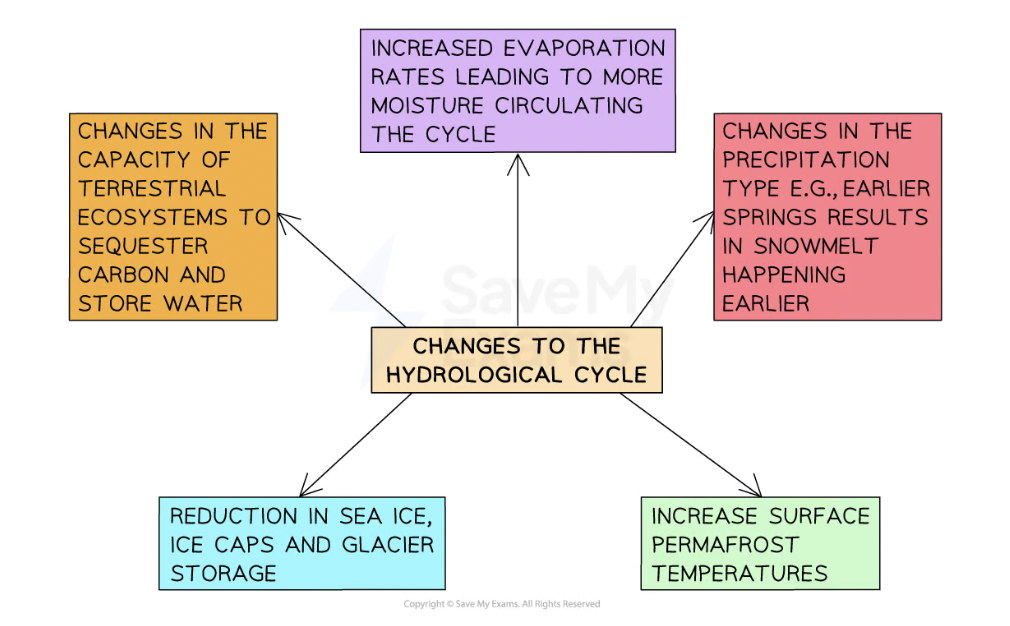
Impacts global climate, ecosystems + hydrological cycle.
Impact of combustion on climate
Higher levels of atmospheric CO2 increase global temps.
Changing temps + therefore salinity levels could affect the thermohaline current.
N. Europe will see more precipitation + S. Europe the opposite- this will change water availability + risk of drought + forest fires in S. Europe.
Impact of combustion on ecosystems
Marine ecosystems threatened by lower oxygen levels, more ocean acidification + food chain changes due to rising temps.
Coastal ecosystems are at risk from sea level rise.
Impact of combustion on hydrological cycle
If precipitation falls as rain and not snow in winter, winter floods can be expected.
A glaciers retreat (melt more than they form each year) there will be an increase in river discharge.