Post-midterm
1/341
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
342 Terms
Where does signal 3 of T cells occur?
Lymph node
When do T cells become effector T cells?
When they have received all three signals
They then go to site of infection to exert their function
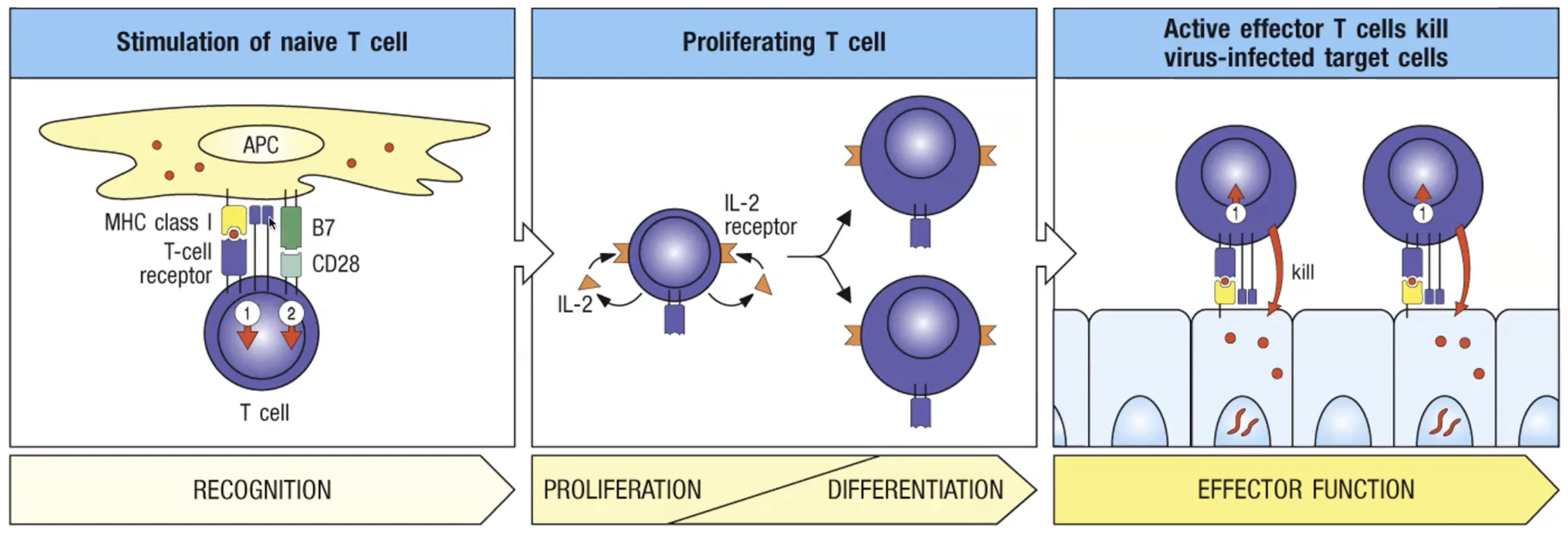
What are the three signals for T cell activation?
1) pMHC:TCR
2) B7:CD28 (B7.1/CD80;B7.2;CD86)
3) Cytokines
What are the effector functions (in general) of CD4+ and CD8+ T cells?
CD4+: activation of macrophrages, B cells, other cells
CD8+: killing of infected target cells, macrophage activation
*memory CD4+ and CD8+ T cells
How do T cells leave to get to the site of infection?
Efferent lymphatic vessels → Thoracic duct → Blood stream (to site of infection)
Where do T cells exert cell-mediated immunity?
At the site of infection
What happens to T cells when the infection is cleared?
Downregulation of T cell activity and Immunological memory
Briefly describe signal 3 of T cells.
Differentiation - cytokines directing T cell differentiation into distinct effector cell types
*all three signals result in intracellular signaling, leading to change (activation, proliferation, survival, differentiation)
Activated T cells proliferate in response to what?
IL-2 cytokine
What can activated T cells differentiate into?
CD4+ helper cells: TH0 into TH1 or TH2 or TFH or TH17 or others
CD8+: cytotoxic T lymphocyte (CTL)
Regulatory T cells
*TH0 are naive T cells, have not received all three signals
How can effector T cells be classified?
The TFs they express
Profile of cytokines they make
Main function in adaptive immunity
Once activated, an effector T cell does not require what to act?
Co-stimulation
To kill an infected cell, it does not require signal 2 again. It will only kill if their TCR is specific to the MHC and peptide
*on nucleated cell therefore MHC I
C
What are the subtypes of CD4 T cells?
TH1
TH2
TH17
TFH
Treg
*different effector cells have different functions, different cytokines secreted and different function
*can impact functionality of other immune cells and clears different microorganisms
What are polarizing cytokines?
Responsible for guiding a T cell toward one of several different effector fates (fate-specifying cytokines)
It is what signal 3 cytokines are referred as
*APCs bind PAMPs via PRRs → cytokine secretion; different PRRs engaged means different cytokines produced
*effector cytokines are produced by differentiated T cells
How does signal 3 exert its effect?
Polarizing cytokines bind their receptor and induce signaling, which occurs through STAT proteins (when phosphorylated act as TFs to go in nucleus and bind to promoters for specific gene)
When specific gene is transcribed, another TF known as master transcriptional regulator is produced (determines functionality of cell)
The MTR binds to response elements on promoter regions of genes coding for specific effector cytokines, which determines activity of activated and differentiated T cell subtype
B
The STAT proteins are waiting to get phosphorylated, which then leads to expression of master transcriptional regulators
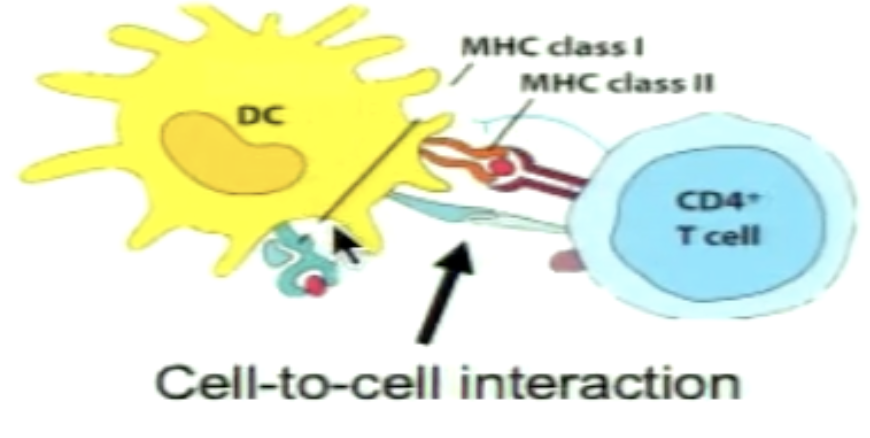
What are the general characteristics of Fas ligand and CD40 ligand?
Expressed on surface of effector T cells (not naive)
included in genes that are induced following the three signals
Crucial for effector T cell function
Transmembrane ligands part of TNF family (cell to cell interaction)
What is Fas ligand?
Expressed on surface of effector CD8+ and TH1
used by CD8+ to exert cytotoxic effect
Binds Fas on surface of infected cells in periphery (site of infection)
What is CD40 ligand?
Expressed by TH1, TH2, TH17, TFH
Binds CD40 on B cells and innate immune cells
Used to activate these target cells
Allows DC licensing and expression of more co-stimulatory moleculaes
CD40 signaling leads to DC licensing and expression of more co-stim molecules
What are the signals for effector CTL generation from naive CD8+ precursors?
1) TCR binds peptide presented by APC on MHC I
2) Co-stimulatory signal transmitted by CD28-B7 (CD80/86) interaction between T cell and APC
3) Provided mainly by IL-2 and other cytokines to some extent (IL-12) inducing proliferation and differentiation into CTL form
What are the specific considerations for CD8+ T cells?
Requires more co-stimulation
IL-2 can be autocrine and paracrine from TH1 or TH17
Requires help of effector CD4+ cells
How can CD8+ cells be activated simply but rarely, and majority of the time?
Simple: by activated DCs that have high co-stim activity (in some viral infections, DCs become infected and sufficiently activated)
Majority: extra help with CD4 effected T cells and license DCs to cross present
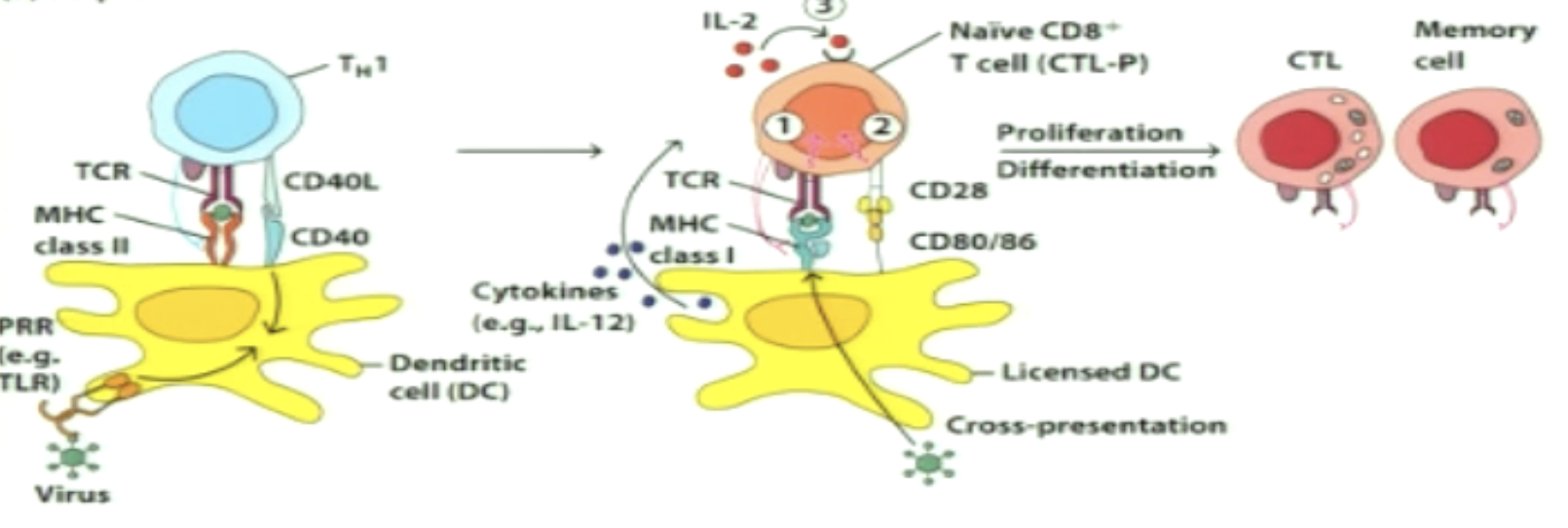
What is sequential activation?
APC becomes further licensed following interaction with a CD4+ cell
signal APC receives through CD40 signaling
Interacts with CD8+ independently
IL-2 produced by CD8+ cells alone induces proliferation (autocrine)
(1 - CD4+ gets activated, 2 - give permission through CD40 ligand binding, 3 - CD8+ activation)
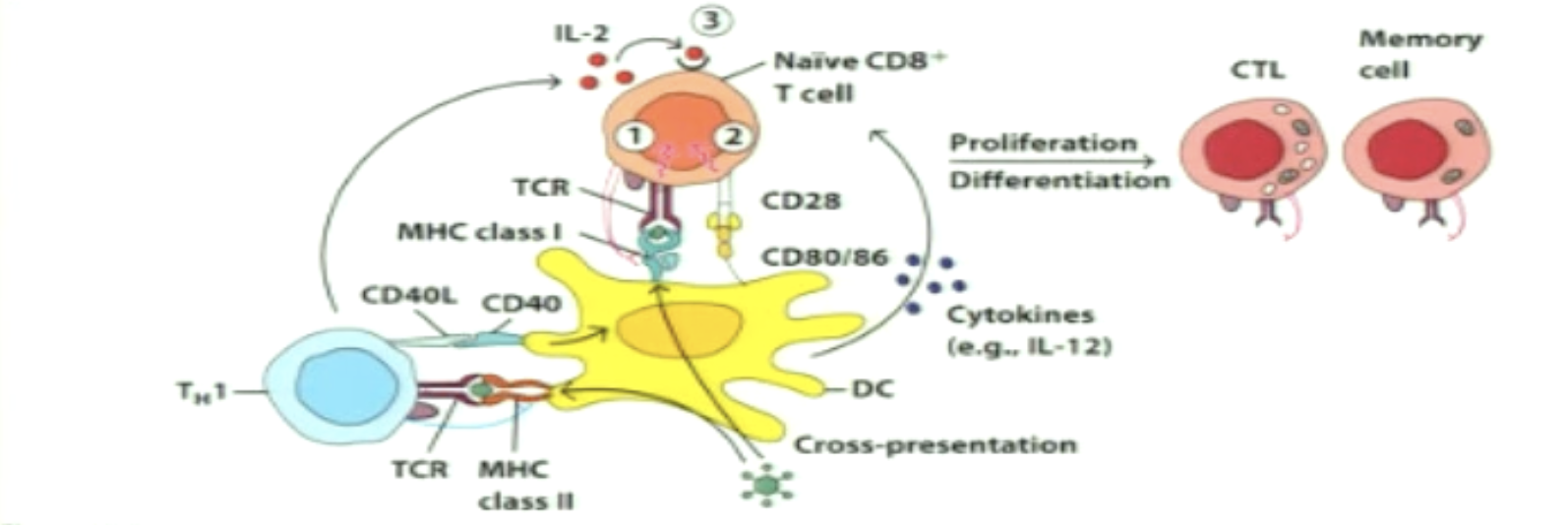
What is simultaneous activation?
APC interacts with both CD4+ and CD8+ cells at the same time (CD4 interaction takes long or CD8 was close by)
First thing that happens is CD4+ activation (must be effector to give CD40 ligand binding to CD40 licensing)
CD40 (DC) - CD40L (T cell)
As CD4+ produces IL-2, it gives some to CD8+ through paracrine
IL-2 still secreted by CD8+ (autocrine) to induce proliferation of CD8+
What needs to happen to CD4+ T cells in order for CD8+ T cells activation to occur?
Find its p:MHC II match
All three signals received
CD40L gets expressed and binds CD40 on APC
What needs to happen to DCs to allow them to present antigen to both CD4+ and CD8+ T cells?
Encounters a PAMP
Travels to lymph node (secondary lymphoid organ) as mature DC
Present antigen on MHC II to CD4+
Gets licensed through CD40 binding
Presents exogenous antigen on MHC I to CD8+ (cross presentation) (increased expression of CD80/86)
What does CD4+ cells secrete and express when activated?
IL-2 (increased production when APC is licensed by activated CD4+)
CD40L
What 4-1BBL and CD70?
An additional molecule that activates CD8+ T cells and binds to 4-1BB (co stim signals for CD8)
CD70 binds to CD27 to provide costimulatory molecules along with B7
What helps generate memory CD8+ T cells?
CD4+ T cells (due to cytokines)
C & D
What are cytotoxic T lymphocytes?
When naive CD8+ cells get activated and differentiates
Leaves lymph node to travel to site of infection
CTLs can kill infected cells in the periphery
interaction of TCR (& CD8 co-receptor) with pMHC I
How do CTLs target infected cells?
*all nucleated cells express MHC I
1) initial interaction via nonspecific adhesion molecules - if pMHC is not a match CTL moves on
2) When CTL recognizes pMHC (infected or cancerous cell), the infected cell dies
How can CTLs induce apoptosis?
Fas-FasL
Granules
Also secrete cytokines to direct immune response
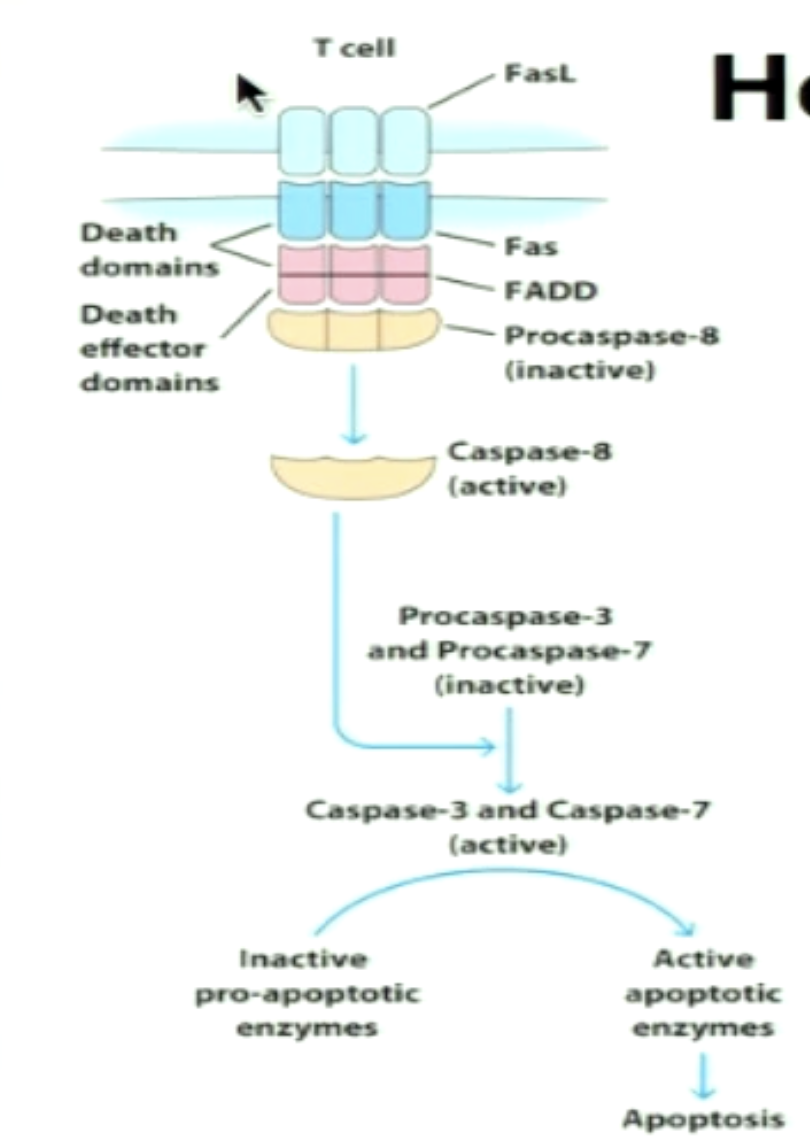
Explain Fas-FasL interaction.
Effector CTL expresses FasL; Infected cells express Fas (after TCR and MHC I interaction)
Signaling cascade involves cleavage of pro-caspases into caspases to lead to apoptosis of infected cell (8→3&7)
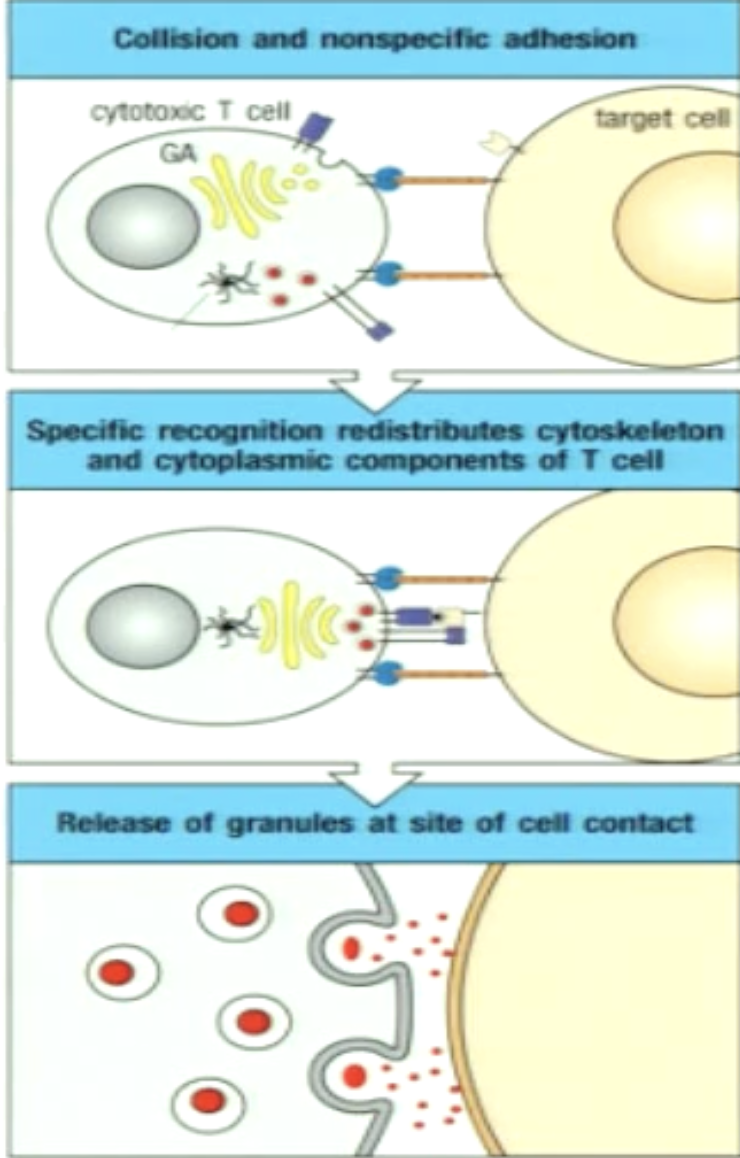
Explain granule mediated killing.
1) CTL first makes contact to target cell via nonspecific adhesion
2) Specific recognition via (CTL) TCR:pMHC (triggers intracellular signaing); reorganization of cytoskeleton and cytplasmic contents
3) Granules released at point of cell contact
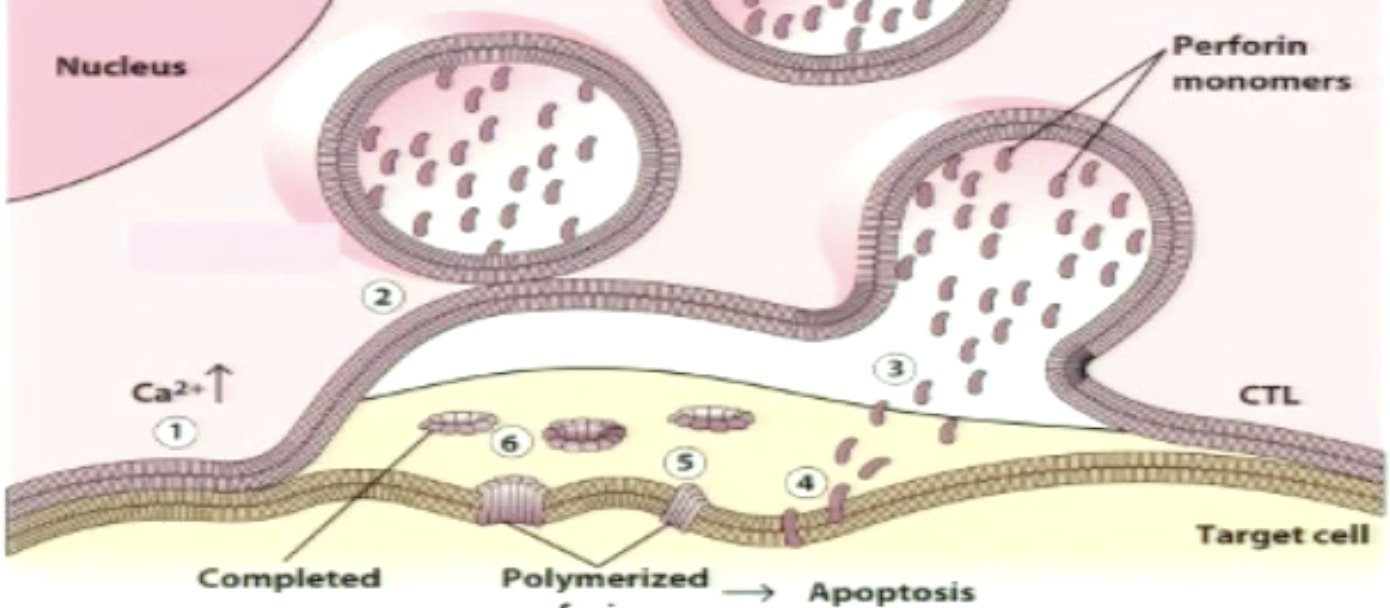
What granules are used in granule-mediated killing? What are their functions?
Perforin: aids in delivering content of granules to cytoplasm of target cell through forming pores
Granzymes/Granzyme B: serine proteases, which activate apoptosis once in the cytoplasm of target cell
Granzyme B: initiates signaling through pro-caspases cleavage into caspases (3), leads to DNA fragmentation and cell death (blebbing where cytoskeleton breaks and membrane bugles indicating apoptosis)
Explain granzyme/perforin-mediate cytolysis.
When stimulated CTLs release granule contents:
Perforin is pore-forming; granzymes are serine proteases
Perforin punches holes in membranes and granzyme enters cytoplasm of target cell to induce apoptosis
Which mechanism does CTL use?
*both converge on various caspase 3 activation leading to apoptosis)
Perforin/Granzyme is fast-acting and CTLs primarily use this
Fas-FasL is slow-acting
Reports show that CTL lytic action is enhanced when both mechanisms operate simultaneously

C

CD8+ T cells in trachea 8 days post infection
Influenza has gone down after 8 days
Conclusion:
CD8+ responsible for reducing virus titer
Takes time (8 days) for adaptive immune response to exert function
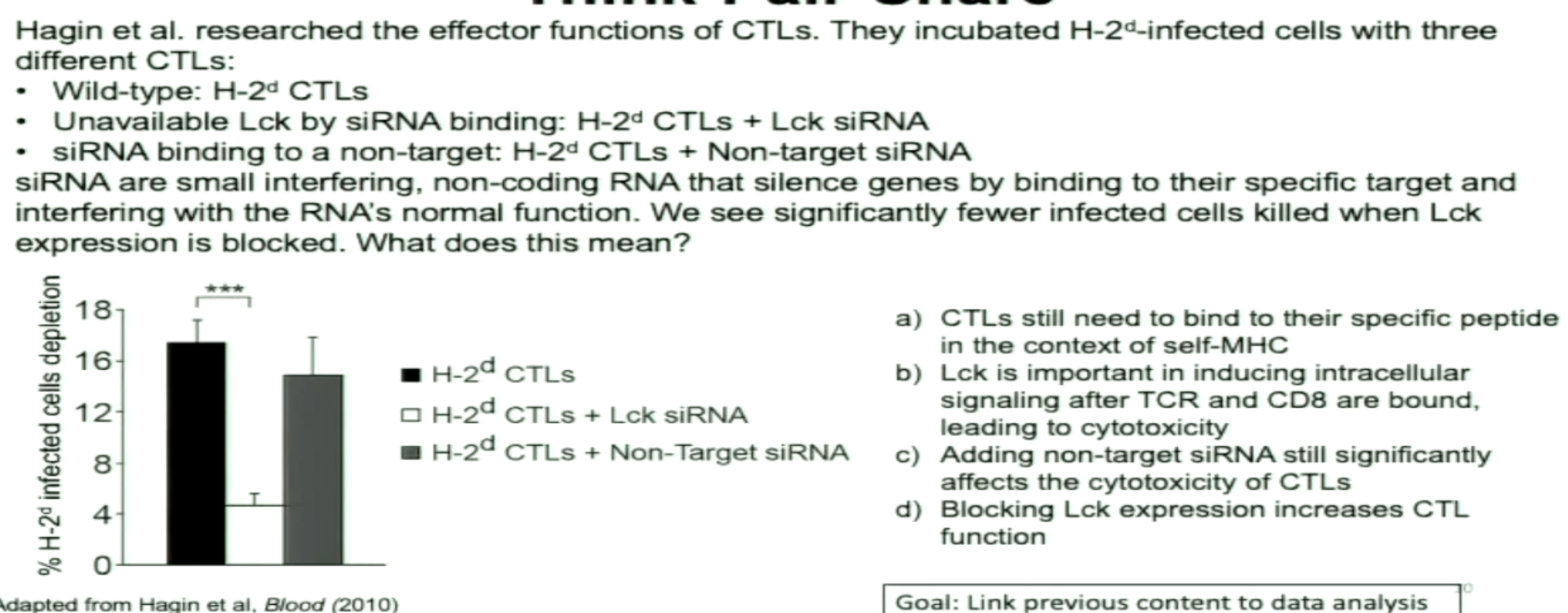
(*** is significant change)
B
Which cytokines do CTLs secrete? What are their function?
IFNgamma (Type II IFN)
Increase MHC I expression in neighbouring cells (increases chance of CTLs finding match)
Activates macrophages and stimulates productive of chemokines that can recruit additional macrophages and CD8+ cells to site of infections
**type I IFN are potent antiviral effects (PRR activation effects); type II has role agaisnt intracellular pathogens, secreted mainly by T cells
What secretes polarizing cytokines?
Secreted by dendritic cell and neighbouring innnate and adaptive immune cells
(fate specifying)
TH1 responses are involved in the response to which infections?
Viruses and Intracellular pathogens (bacteria and parasites)
CTLs are involved too
TH2 responses are involved in the response to which infections?
Parasites/helminths and other extracellular pathogens
*facilitates tissue repair
Dysregulated TH2 response are involved in allergies or asthma
TH17 responses are involved in the response to which infections?
Extracellular bacteria and fungi (and autoimmunity)
TFH responses are involved in what?
Activating B cells in the lymph node
In response to all types of pathogens
Based on what type of infection, the ___ response is dominated by ___
Cell-mediated; one of the T cell subsets
What are the key effector molecules and cytokines for CTLs?
Perforin
Granzyme B
Fas ligand
IFNgamma (Type II IFN)
(p:MHC I)
What are the key effector molecules and cytokines for TH1 cells?
IFNgamma
CD40 ligand
Fas ligand
(p:MHC II)
What are the key effector molecules and cytokines for TH2 cells?
IL-4
IL-5
IL-13
CD40 ligand
(p:MHC II)
What are the key effector molecules and cytokines for TH17 cells?
IL-17
IL-22
CD40 ligand
(p:MHC II)
What are the key effector molecules and cytokines for Treg cells?
IL-10
TGF-B (Beta)
(p:MHC II)
What is cross-regulation? Give some examples.
During response to an infection, there will be a combination of different T cells but one will dominate
IL-4 (secreted by TH2) inhibits TH1 differentiation
IFNgamma (secreted by TH1) inhibits TH2 proliferation
IL-4 or IFNgamma inhibits TH17 differentiation
These effector cytokines can help reinforce the predominant subtype they are part of
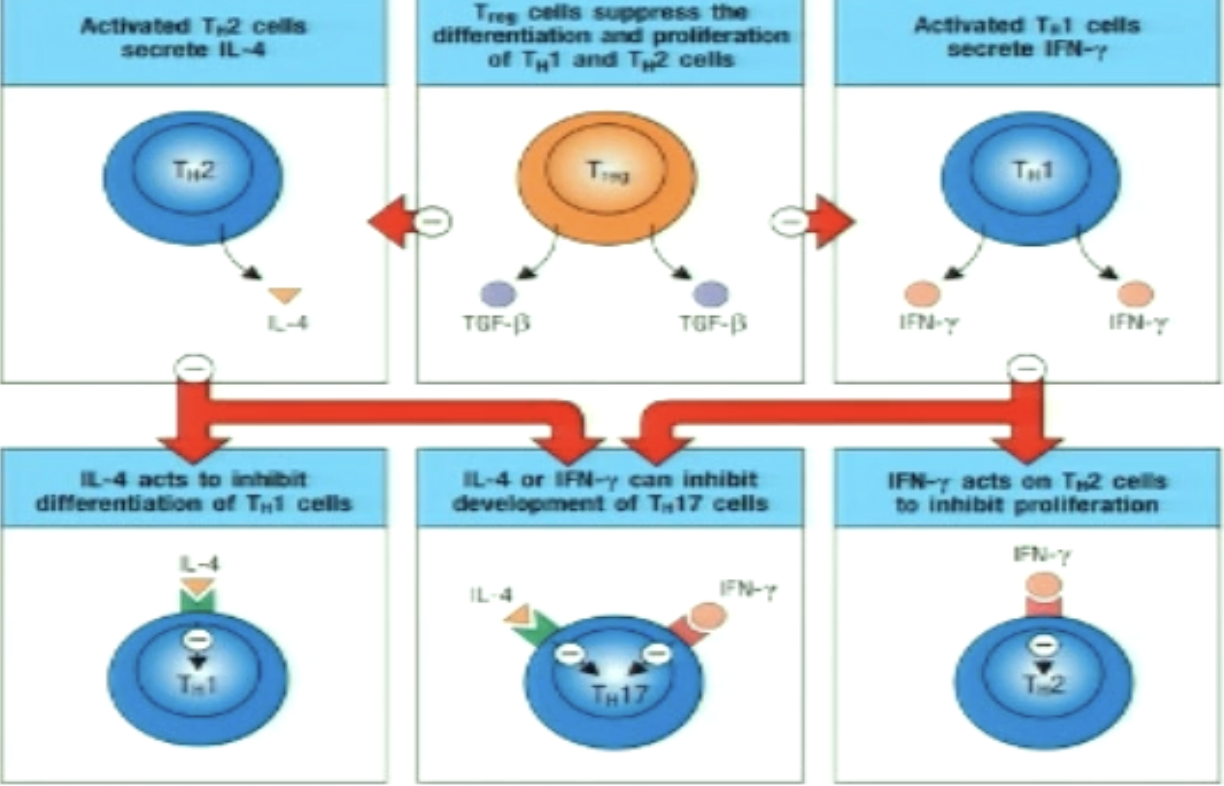
Explain TH1/TH2 cross-regulation.
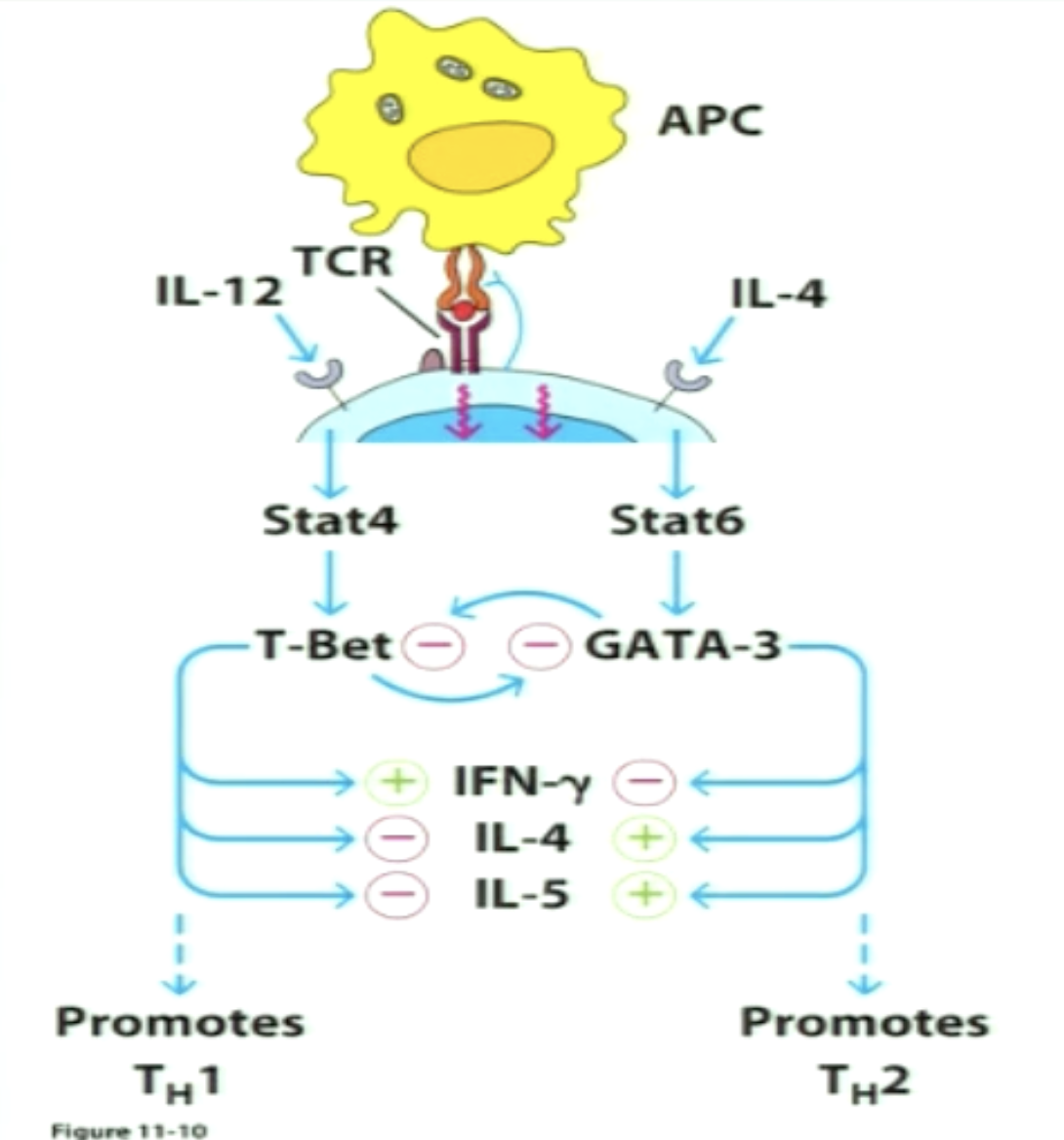
Master transcriptional regulators commit T cells to one subset or the other
T-Bet (master transcriptional regulator for TH1) suppresses TH2 pathway gene expression
positively regulate IFNgamma and negatively regulates IL-4 and IL-5
GATA3 (MTR of TH2) suppresses TH1 pathway gene expression
negatively regulate IFNgamma and positively regulates IL-4 and IL-5
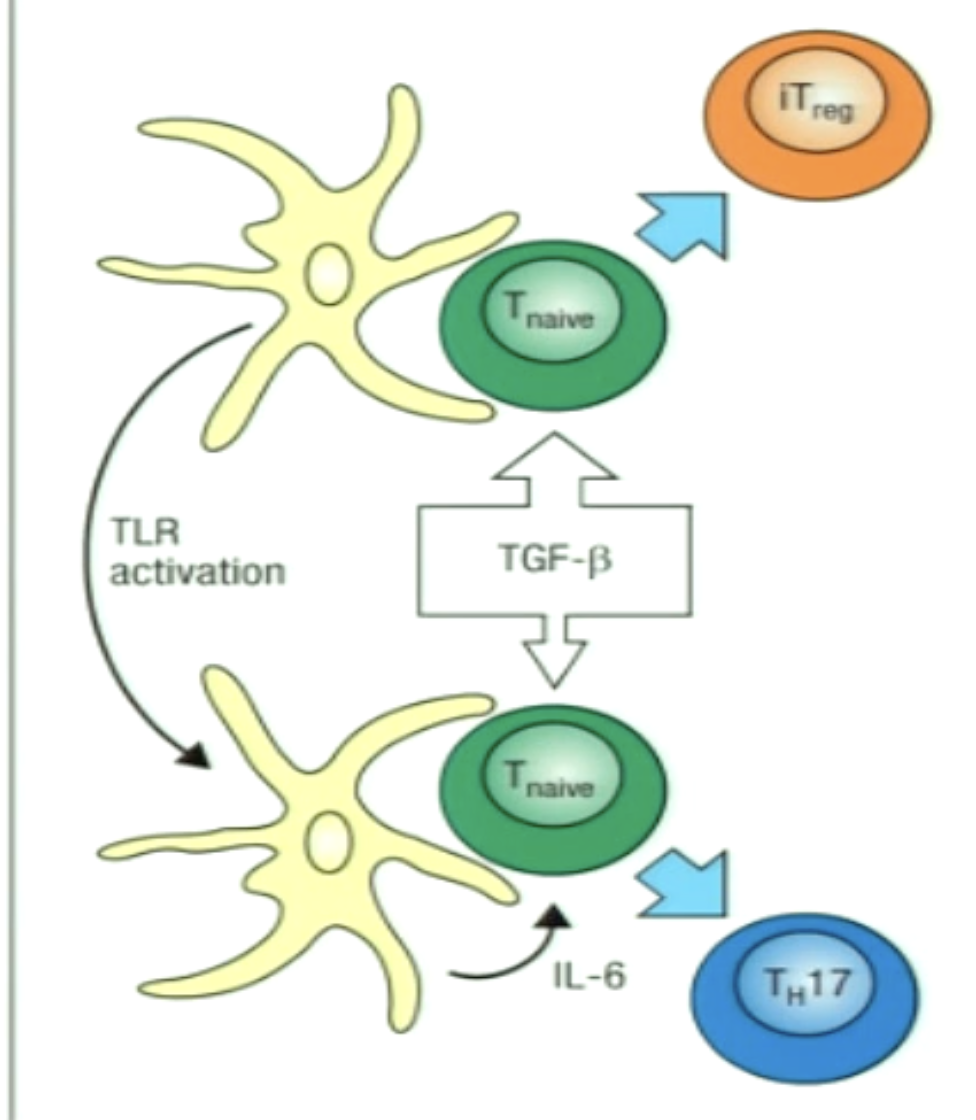
Explain the decision point of TH17/Treg.
TGF-B is a key cytokine for differentiation of both subsets
IL-6 is the switch which induces TH17 subset differentiation instead
A balance between the two is beneficial:
Normal state can favour development of iTreg population to keep inflammation down
Infection (leading to IL-6 production) would stimulate a more antibacterial TH17 differentiation
Which T cells leave the lymph node and migrate to the site of infection? Which ones stay in the lymph and activated B cells?
Leave: TH1, TH2, TH17
Stay: TFH
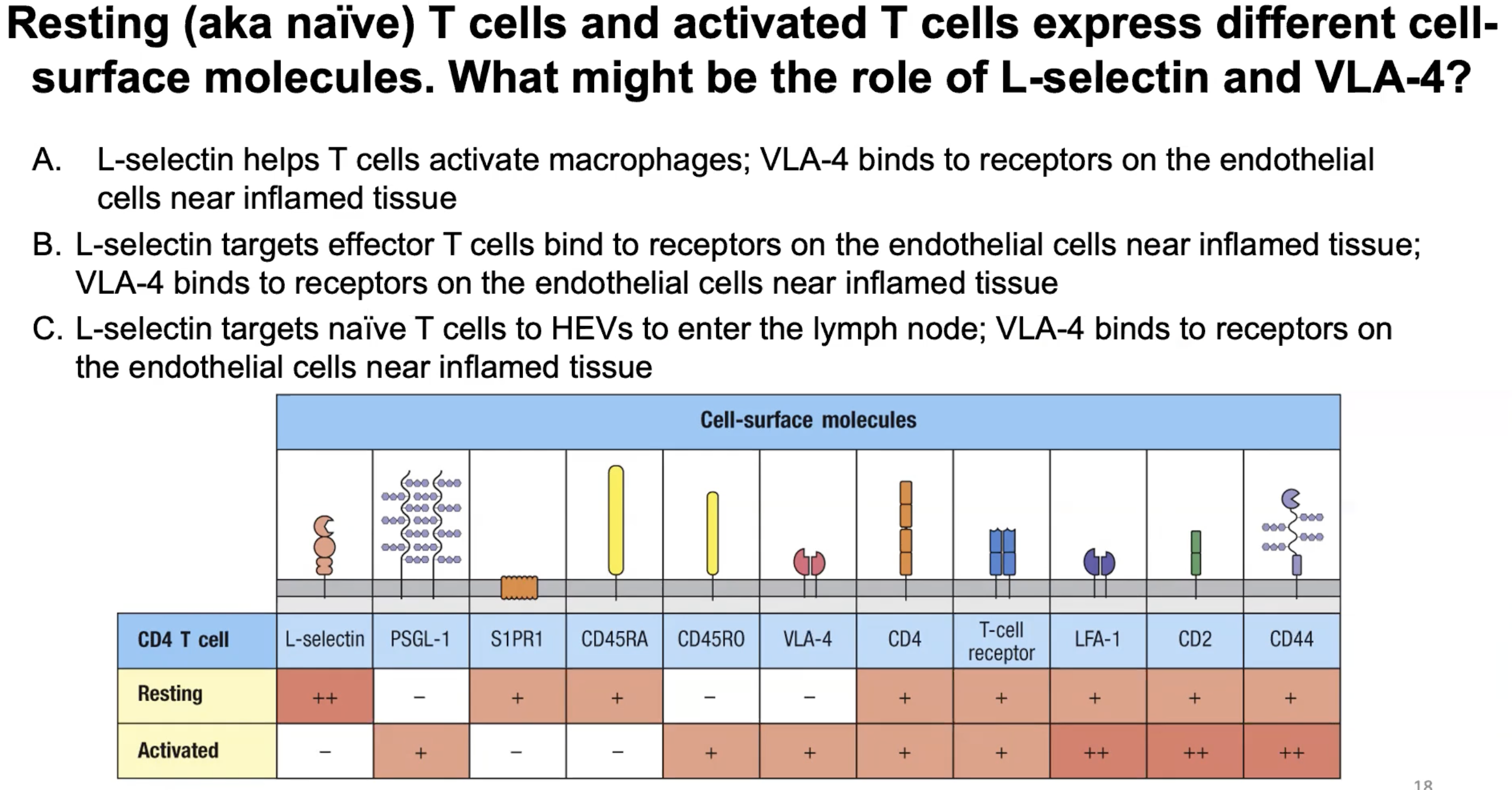
Different ___ molecules are expressed by naive vs effector T cells since they ___
Cell surface molecules; travel to different locations in the body
*effector T cells express molecules that target them to specific tissues (skin vs gut) depending on the site of infection
How is the travel of effector T cells partly determined by?
The travel is partly determined by which lymph node they get activated
Ex: if they are activated in a lymph node that drains the skin, the effector T cells will up-regulate homing that send them to the skin)
Which polarizing cytokines/signal 3 are used for TH1?
IFNgamma
IL-12
Which effector cytokines are secreted by TH1?
IFNgamma
What is the master transcriptional regulatory of TH1?
T-bet
What is the function of TH1 in cellular immunity?
Macrophage activation
Activation and differentiation of naive cytotoxic T lymphocyte precursors into effector CTL (through CD40L binding)
Which transcription factors are needed for TH1?
STAT 1 and STAT 4
(signal 3 cytokines phosphorylate them)
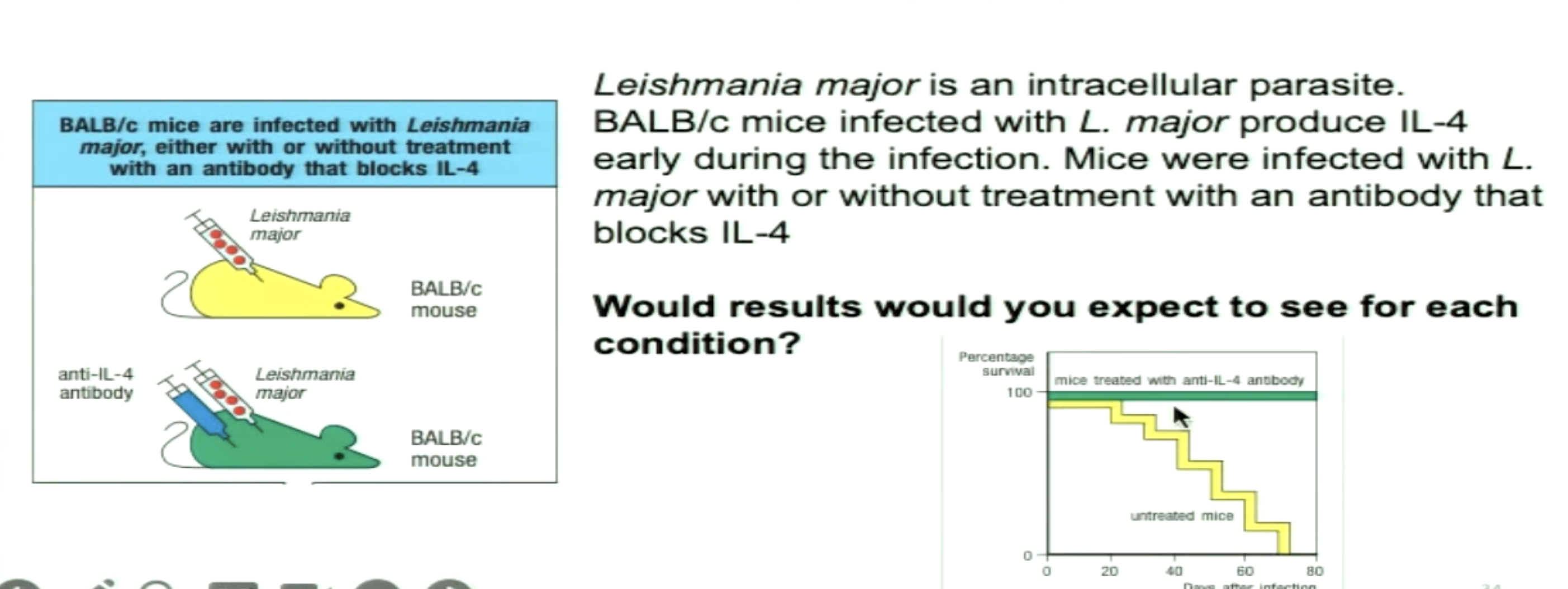
Why would BALB/c mice with anti-IL-4 survive?
a) BALB/c mice would normally induce a TH2 response and with anti-IL-4 antibody, that would inhibit differentiation of naive T cells to TH2 and instead develop a TH1 response
b) Anti-IL-4 antibodies would lead naive T cells to differentiate into TH17 which are important to clear intracellular parasites
c) BALB/c mice would normally induce a TH1 response and with anti-IL-4 antibody, that would inhibit differentiation of naive T cells to TH1 and instead develop a TH2 response
a) BALB/c mice would normally induce a TH2 response and with anti-IL-4 antibody, that would inhibit differentiation of naive T cells to TH2 and instead develop a TH1 response
*TH1 to be dominant to clear intracellular infections
What type of response are TH1 cells?
Type I response
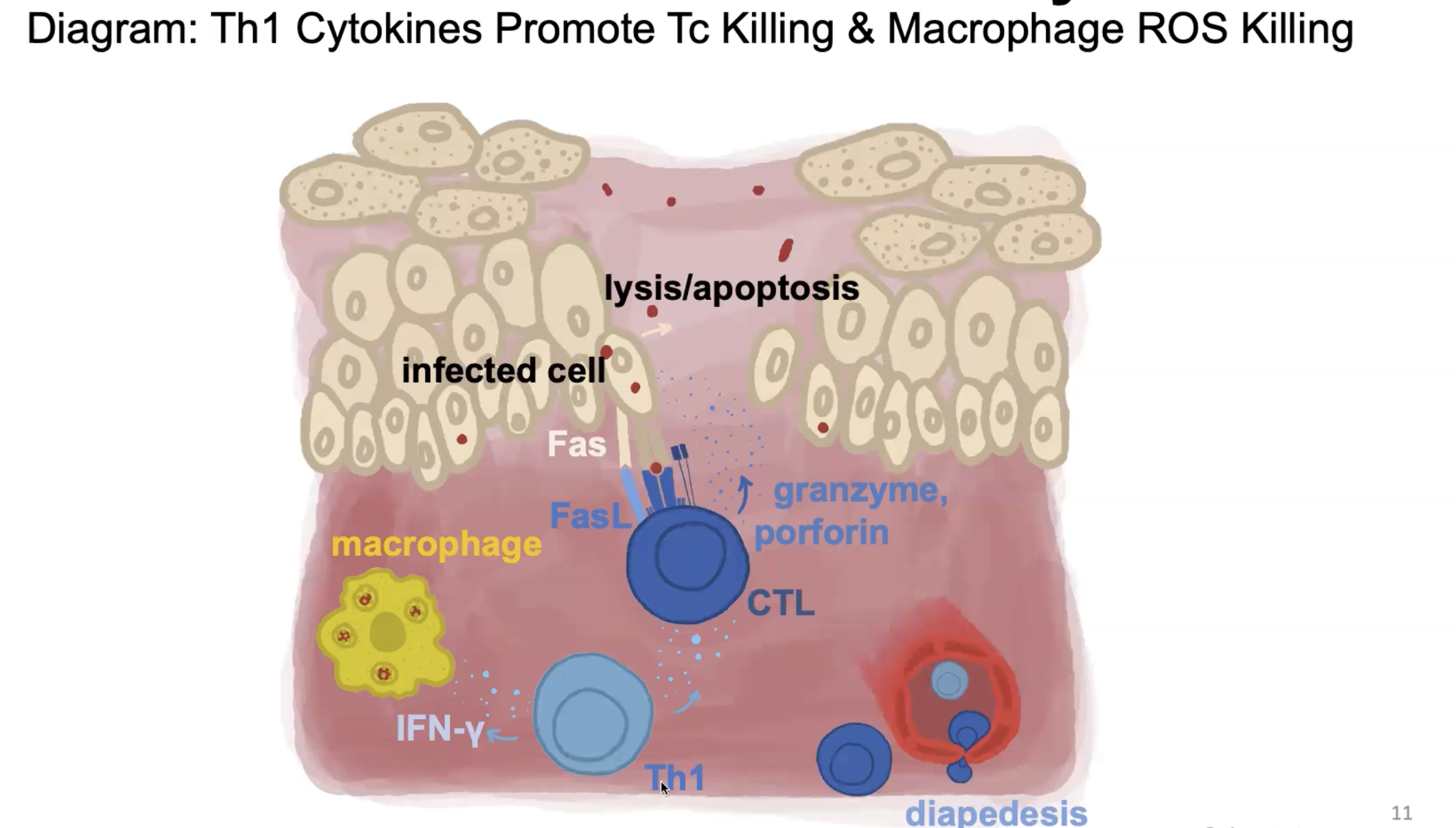
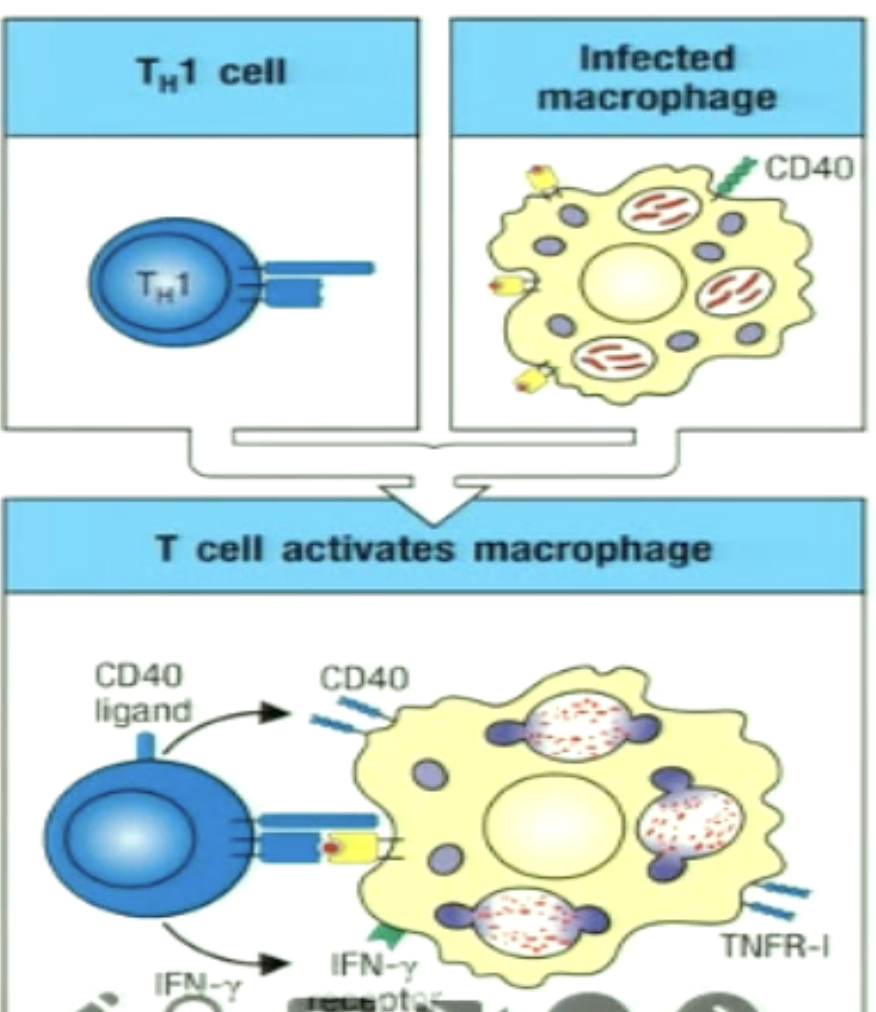
How can TH1 cells activate macrophages?
Some pathogens persist in macrophages (inhibit fusion of phagosome and lysosome; prevent acidication so lysosomal proteases cannot activate)
The TH1 cell recognizes pMHC II on the surface and uses CD40L to bind to CD40
Secretes IFNgamma
Boosts macrophage antimicrobial activity and production of TNFa from macrophage
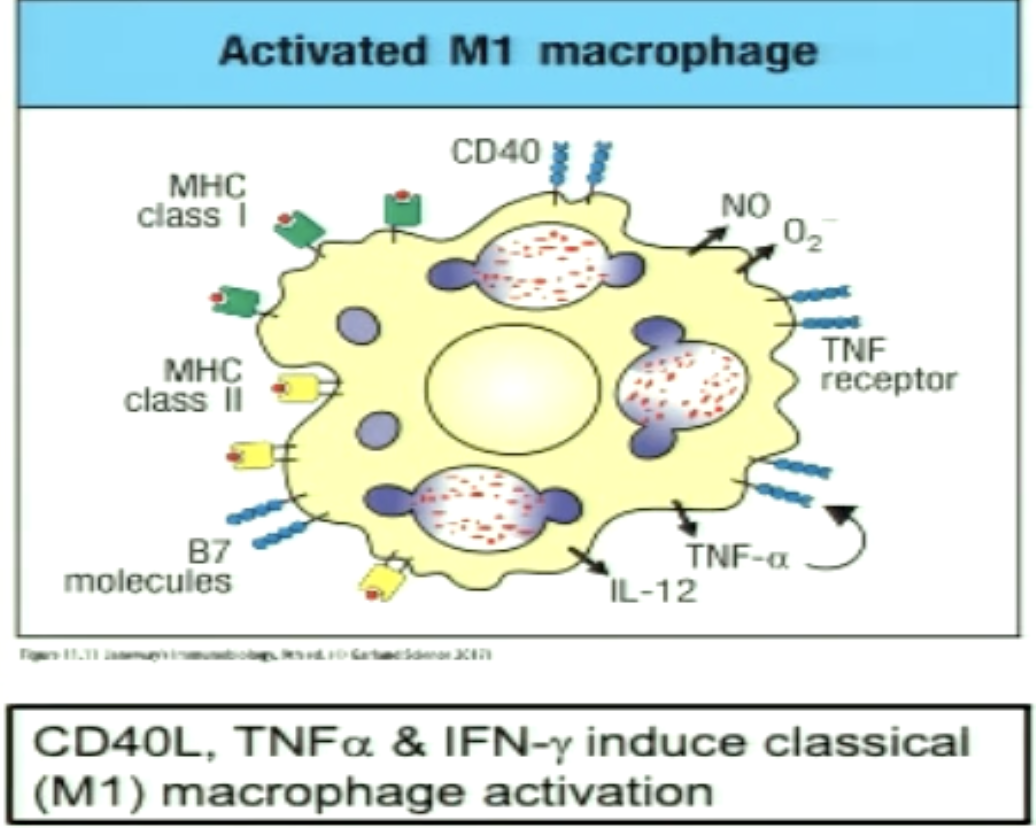
What is an activated M1 macrophage (classically activated macrophage)?
Function induced in the context of TH1 and have a boost to their antimicrobial mechanisms (ROS)
TNFa is secrete which provides autocrine signaling as survival signaml to macrophage
CD40L binding activates macrophages and increases expression of IL-12 (part of signal 3 for TH1)
TNFa and IFNgamma increase expression of MHC I and II, CD40, B7 molecules, and IL-12 which further activates TH1 cells (feed forward loop)
increases chance of TH1 and CTLs to find match
*IFNgamma from effector CTLs can also activate M1 macrophages
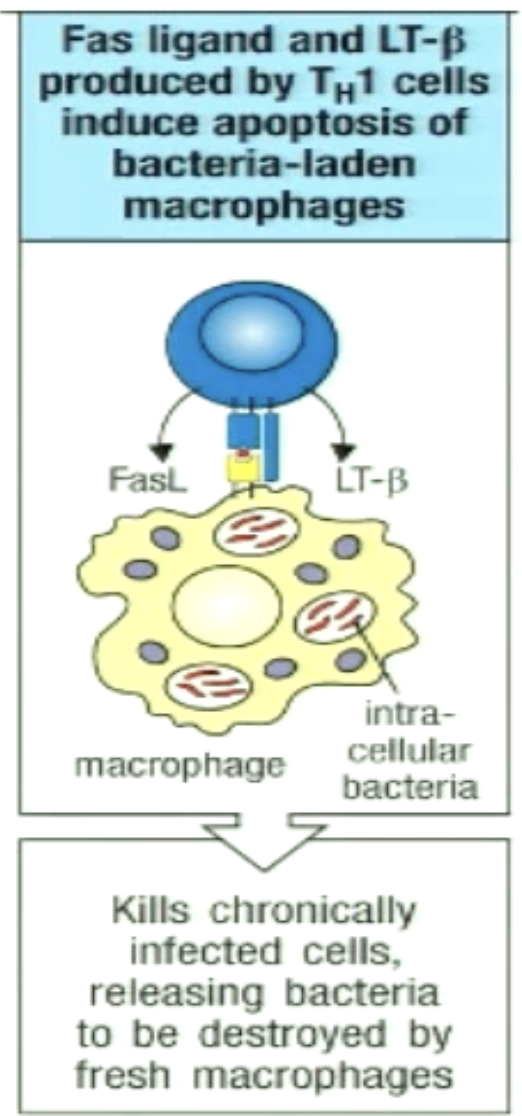
How can TH1 kill infected macrophages?
For chronically infected macrophages
TH1 cells recognize pMHCII on infected macrophage
FasL on TH1 cell binds Fas on infected cells to trigger apoptosis
Bacteria released can be phagocytosed by freshly recruited macrophages
How can TH1 cells help CD8+ cells?
TH1 can secrete IL-2 and stimulate CD8+ proliferation and differentiation
Occurs in lymph node
CTLs can recognize via pMHC I and kill infected macrophages
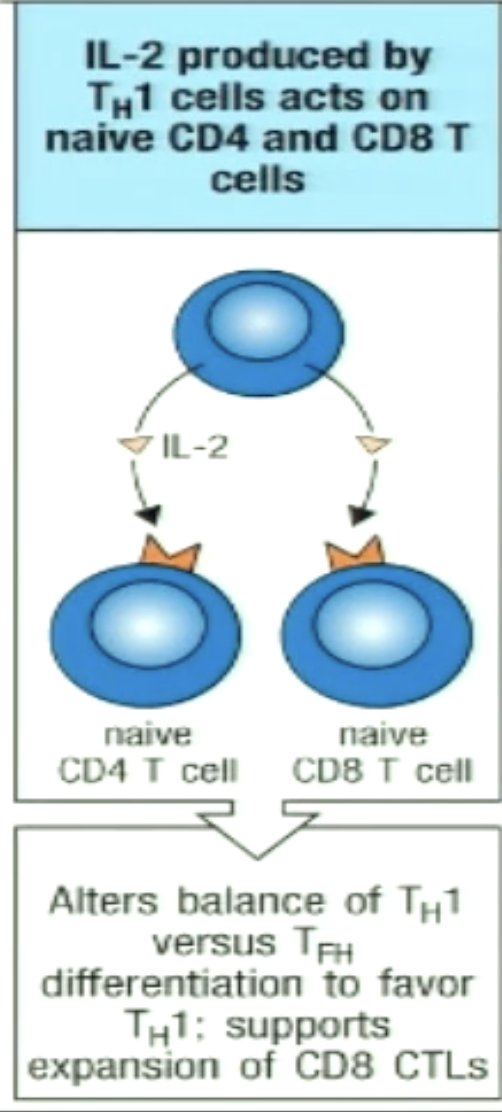
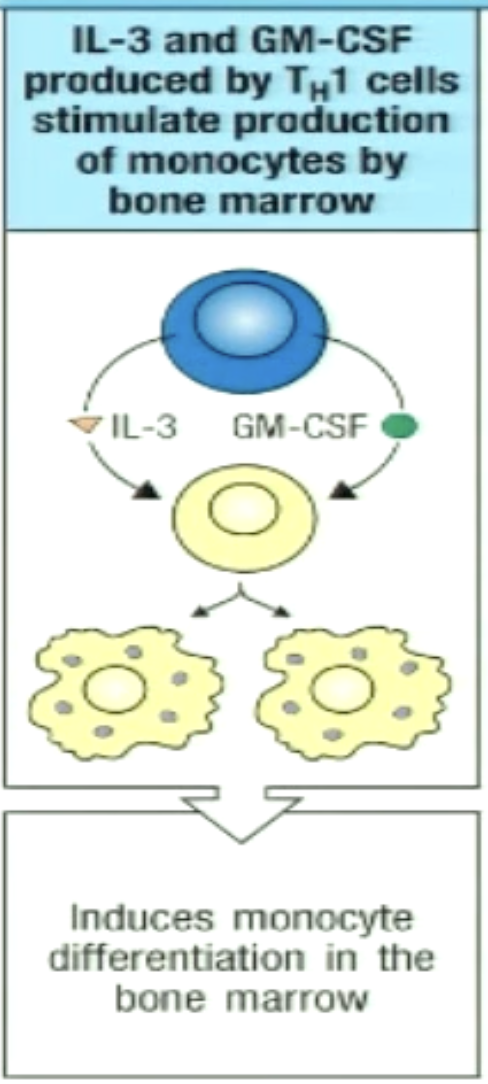
How can TH1 stimulate increased differentiation of monocytes in bone marrow?
TH1 secrete IL-3 and GM-CSF which circulate in the blood and act on precursors in the bone marrow (endocrine)
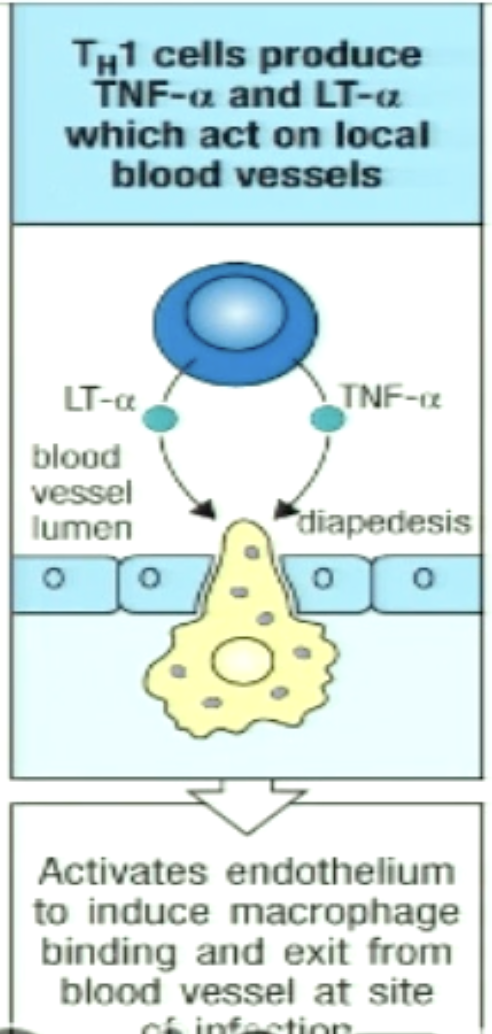
How can TH1 change expression of adhesion molecules on neighbouring endothelium to recruit more macrophages?
TH1 cells secrete cytokines that induce these changes
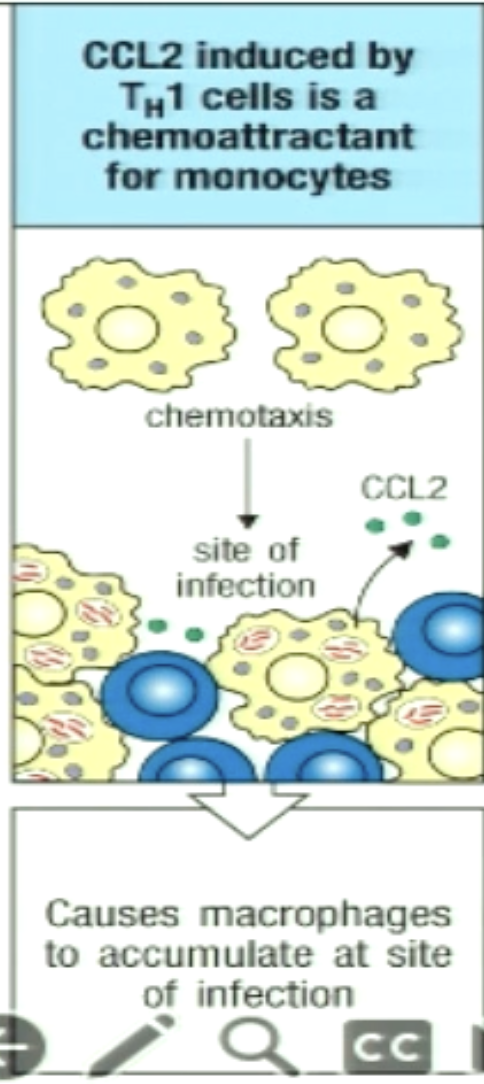
How can TH1 recruit macrophages by chemotaxis?
TH1 cells secrete chemokine CCL2 that attract macrophages to the site of infection

C
What type of response do TH2 cells have?
Type 2 response
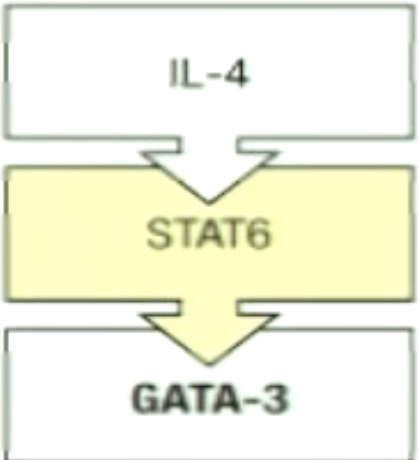
What is the third signal for TH2?
IL-4
What are the effector cytokines for TH2?
IL-4, IL-5, IL-13
What is the master transcriptional regulator for TH2?
GATA-3
What do TH2 cells target/activate when responding to their pathogens?
In response to helminths, they activate eosinophils, mast cells, basophils, and macrophages
Which transcription factors are needed for TH2?
STAT 6
What is the weep and sweep method used for?
Sometimes the helminth is not cleared (chronic infection), so the worm burden is reduced through weep and sweep
Which antibodies do TH2 cells recruit?
Recruits IgE antibodies, as they are important for immune responses against helminths
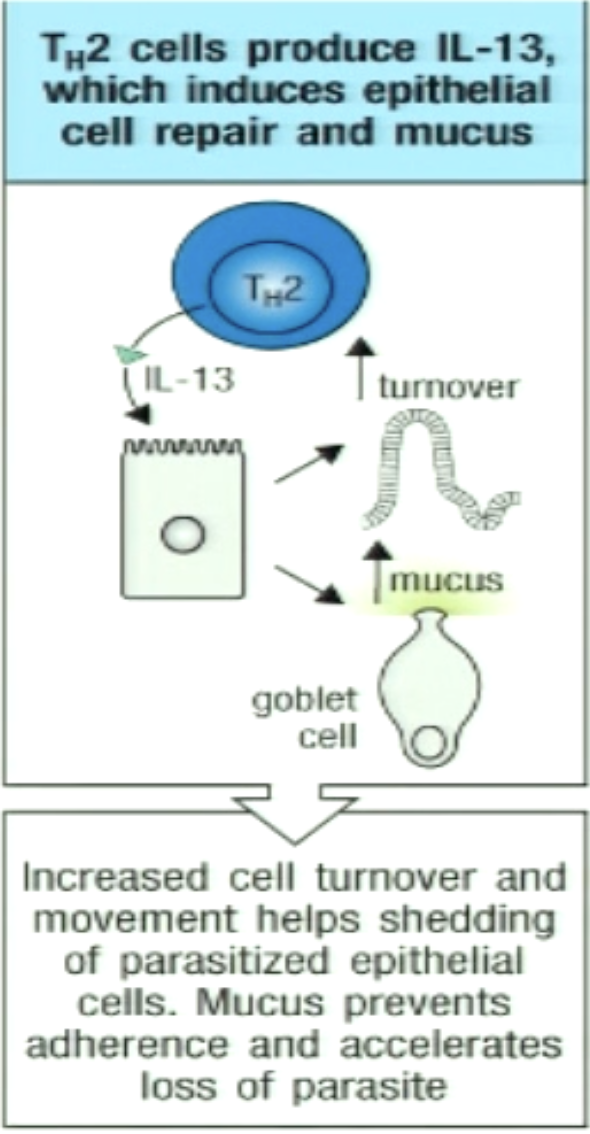
How can TH2 promote cell turnover and mucous production?
IL-13 can increase mucus production by goblet cells and increase turnover of epithelial tissue
parasites have a harder adhering to gut and colonizing
The weep in weep and sweep response
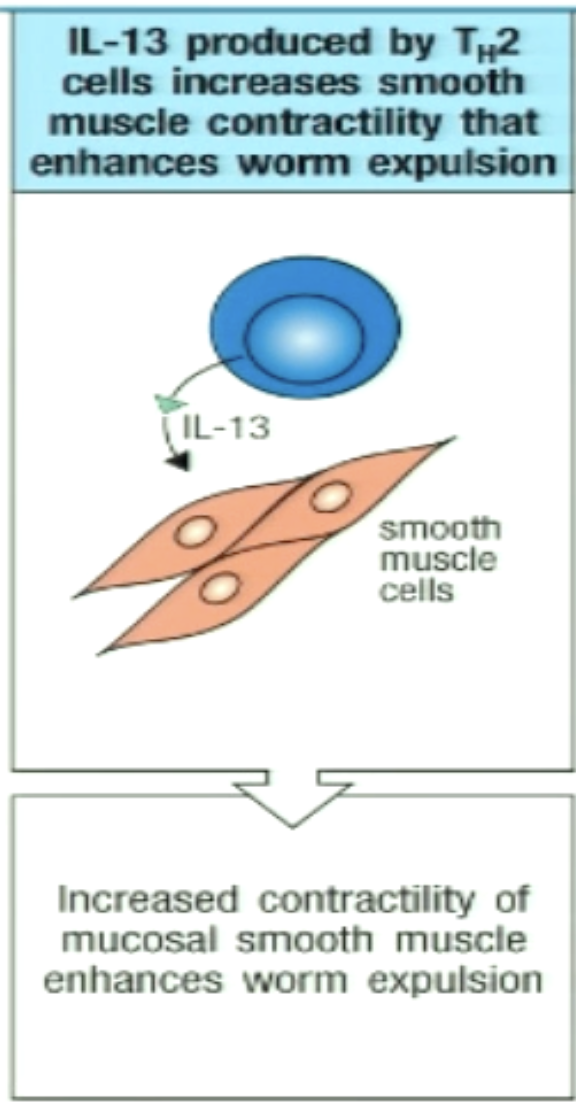
How can TH2 enhance worm expulsion?
IL-13 can stimulate smooth muscle cells to contract
can lead to worm expulsion (physical removal of worm)
The sweep in weep and sweep response
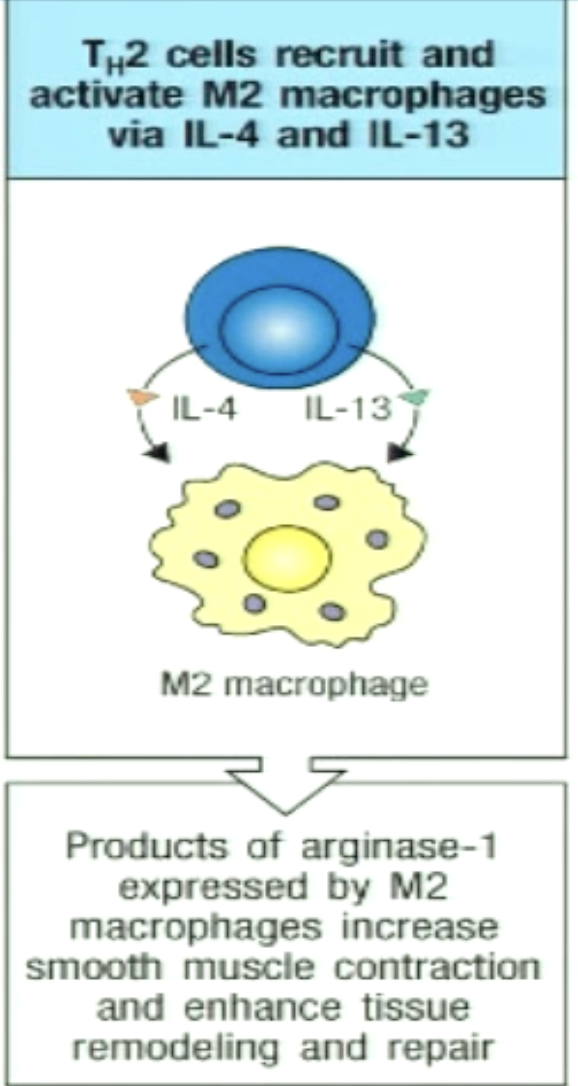
How can TH2 recruit and activate M2 macrophages?
M2 macrophage: alternatively activated macrophage
IL-4 and IL-13 important for M2 macrophage
M2 macrophages aid tissue repair and participate in worm killing and expulsion (increase smooth muscle contraction)
M2 mac can form granulomas to entrap worms
M2 mac release toxic mediators directly onto the worm by antibody-dependent cell-mediated cytotoxicity (ADCC)
killing of antibody coated target cells by cell with Fc receptors (receptors bind to antibodies) - mediated by NK
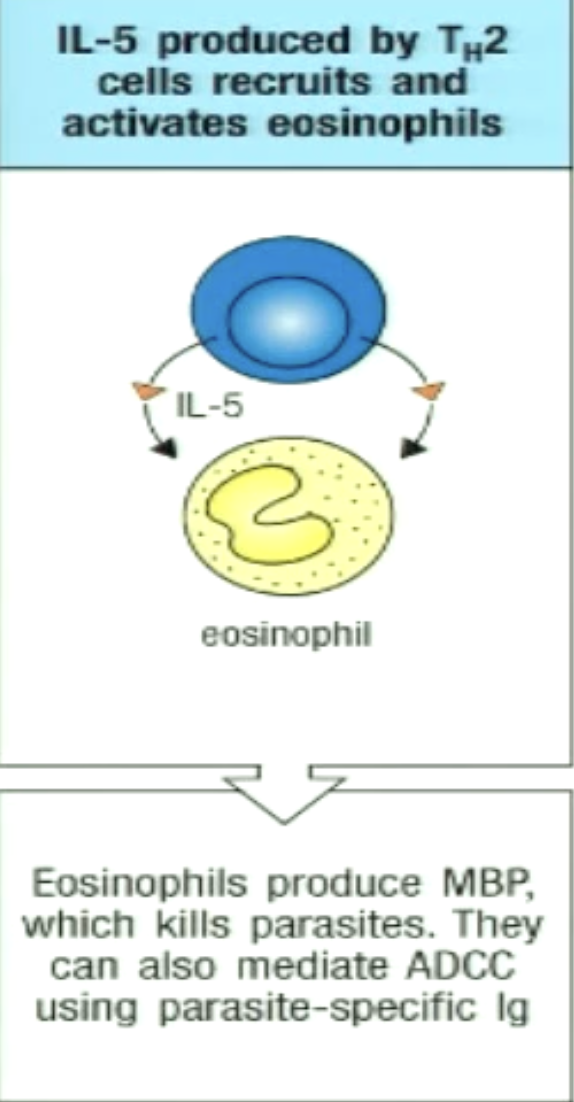
How can TH2 play a role in eosinophil activation?
IL-5 activates, recruits, and enhances eosinophil differentiation
Eosinophil granules contain major basic protein (MBP) which can kill parasites
IL-4 and IL-13 lead to IgE (opsonin) generation
bind antigens on parasites, eosinophils express receptors that recognize the Fc portion of IgE
eosinophils can then specifically target pathogen and degranulate to kill it
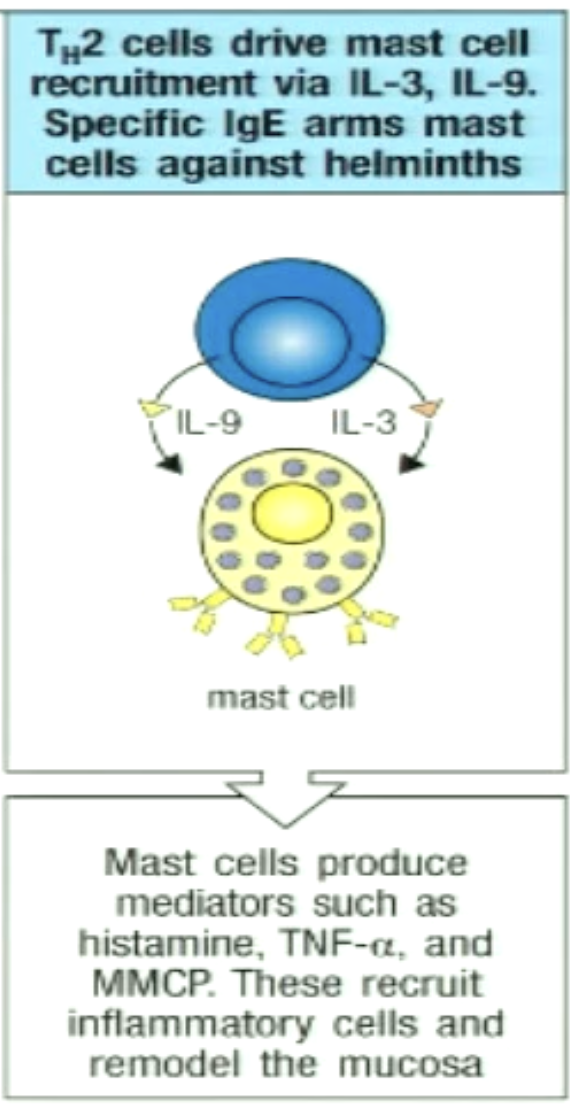
How can TH2 play a role in mast cell activation?
Cytokines activate mast cells, which contain granules of histamine and other molecules
increases vascular permeability
increases intestinal motility
increases recruitment of inflammatory cells
IgE Abs coat parasite, mast cells express receptors that recognize the Fc portion of IgE
How are basophils involved in TH2 effector functions?
Secrete IL-4 and IL-13
Activate goblet cells
Allow vasodilation
Binds to IgE and releases histamines
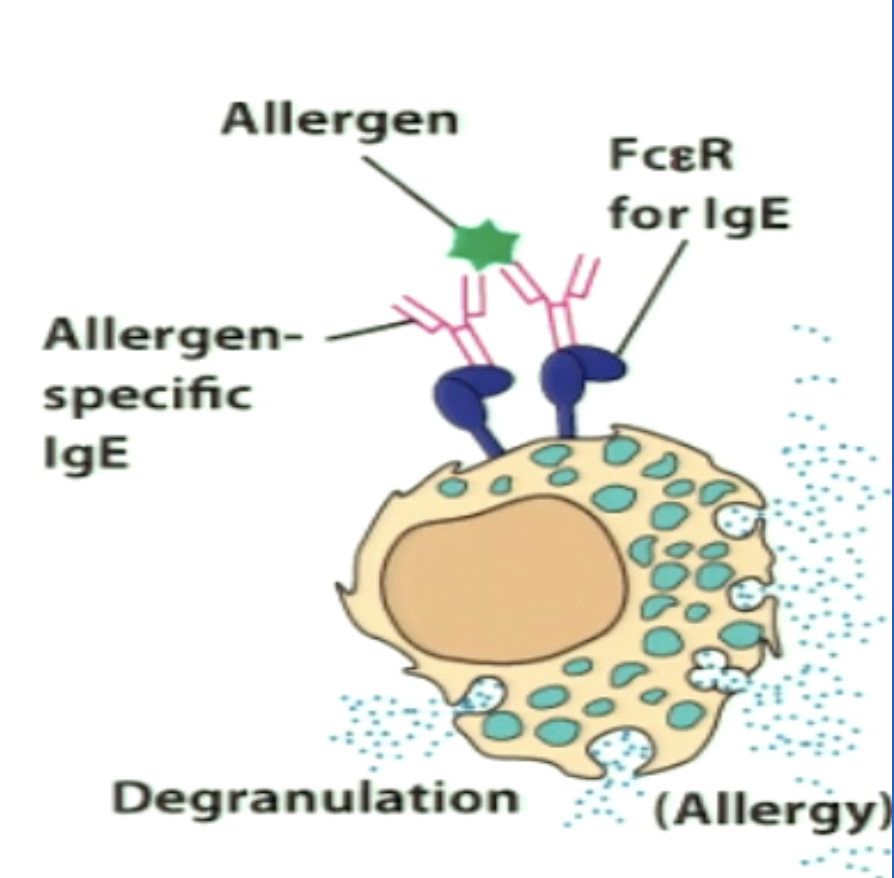
Explain the immune response to allergies.
*Type 2 response
Allergens enter via mucosal tissues and induce TH2 response
IL-4 and IL-13 induce IgE generation
Allergies are initiated by an interaction between an IgE Ab and an Ag
IgE Abs bind to mast cells or basophils and induce degranulation
Granule contents released include histamine, proteases, chemokines (inflammatory)
Act on surrounding tissues and cells to cause symptoms
Free circulating IgE is usually low in concentration in blood serum
Healthy individuals only make IgE in response to parasites

B
*IgE part of humoral immunity
What is signal 3 of TH17 cells?
TGF-B
IL-6
IL-23
What effector cytokine is secreted by TH17?
IL-17 and IL-22
What is the master transcriptional regulator for TH17?
RORgamma T
What cells does TH17 interact with? What does it respond to?
Enhances neutrophil responses and mucosal immunity
respond to extracellular bacteria and fungi
Pro-inflammatory response and involved autoimmune disorders (from dysregulation)
psoriasis, inflammatory bowel disease, asthma, arthritis, MS
What transcription factor is used for TH17?
STAT 3
What type of response do TH17 cells have?
Type 3 response
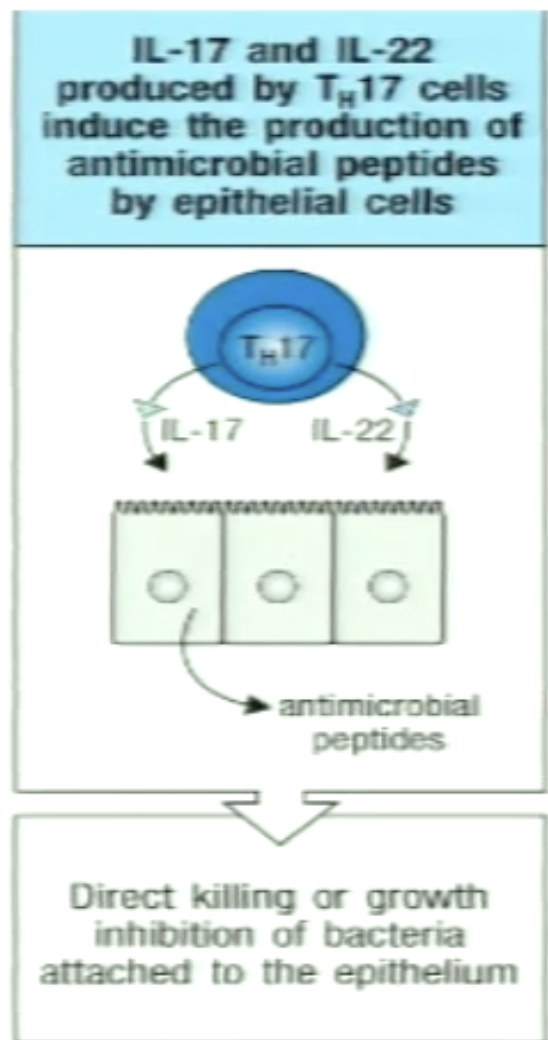
How can TH17 induce production of antimicrobial peptides?
IL-17 and IL-22 induce epithelial cells to produce antimicrobial peptides
Can contribute to killing/slowing replication of bacteria/fungi
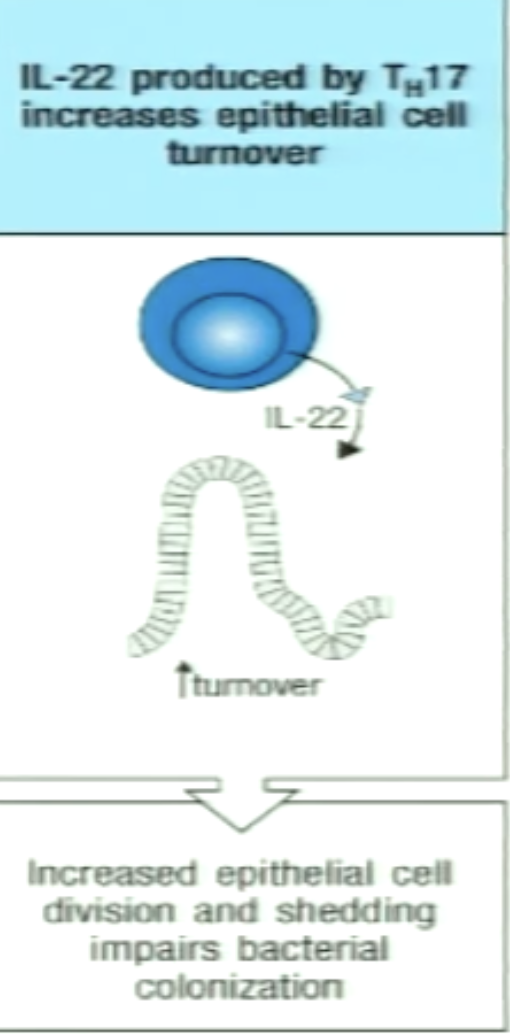
How can TH17 increase epithelial turnover?
IL-22 increases division and shedding of epithelial cells
Hinders bacterial/fungal growth
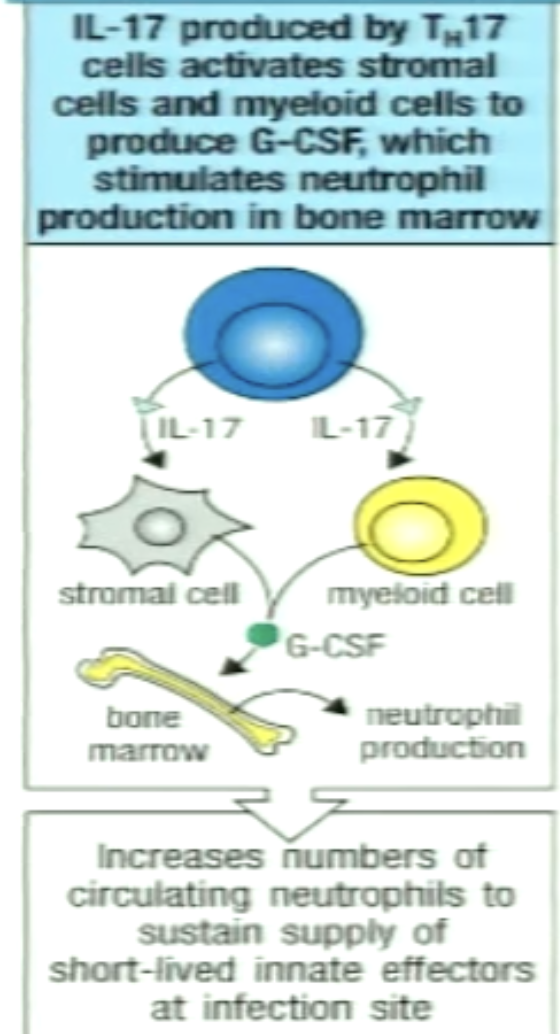
How can TH17 induce other cells to produce G-CSF?
IL-17 acts on stromal and myeloid cells
Secretes G-CSF (cytokine)
G-CSF enters circulation (endocrine) and targets bone marrow precursors to differentiate intro neutrophils