Orgo CH1-12 ALL Practice Problems
1/222
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
223 Terms
What is the ground-state electron configuration of each of the following elements:
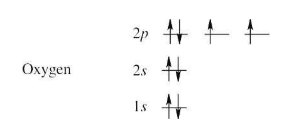
(a) Oxygen
(b) Nitrogen
(c) Sulfur
(a) | To find the ground-state electron configuration of an element, first locate its atomic number. For oxygen, the atomic number is 8; oxygen thus has 8 protons and 8 electrons. Next, assign the electrons to the proper energy levels, starting with the lowest level. Fill each level completely before assigning electrons to a higher energy level. (image attached) Notice that the 2p electrons are in different orbitals. According to Hund’s rule, we must place one electron into each orbital of the same energy level until all orbitals are half filled. Remember that only two electrons can occupy the same orbital, and that they must be of opposite spin. A different way to represent the ground-state electron configuration is to simply write down the occupied orbitals and to indicate the number of electrons in each orbital. For example, the electron configuration for oxygen is 1s2 2s2 2p4. | |
(b) | Nitrogen, with an atomic number of 7, has 7 electrons. Assign these to energy levels. The more concise way to represent ground-state electron configuration for nitrogen: 1s2 2s2 2p3 | |
(c) | Sulfur has 16 electrons. | |
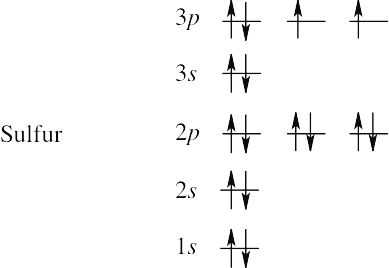 | ||
1s2 2s2 2p6 3s6 3p4 |
How many electrons does each of the following biological trace elements have in its outermost electron shell?
(a) Magnesium
(b) Cobalt
(c) Selenium
The elements of the periodic table are organized into groups that are based on the number of outer-shell electrons each element has. For example, an element in group 1A has one outer-shell electron, and an element in group 5A has five outer-shell electrons. To find the number of outer-shell electrons for a given element, use the periodic table to locate its group. | ||
(a) | Magnesium (group 2A) has two electrons in its outermost shell. | |
(b) | Cobalt is a transition metal, which as two electrons in the 4s subshell, plus seven electrons in its 3d subshell. | |
(c) | Selenium (group 6A) has six electrons in its outermost shell. | |
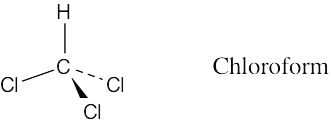
Draw a molecule of chloroform, CHCl3, using solid, wedged, and dashed lines to show its tetrahedral geometry.
A solid line represents a bond lying in the plane of the page, a wedged bond represents a bond pointing out of the plane of the page toward the viewer, and a dashed bond represents a bond pointing behind the plane of the page.
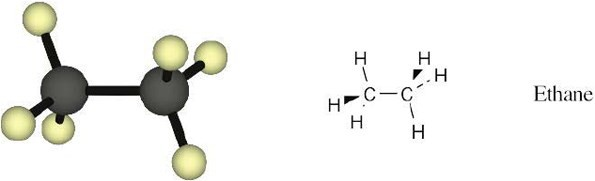
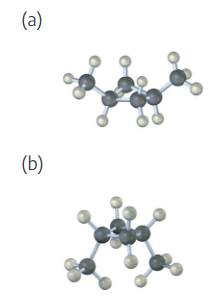
Convert the following representation of ethane, C2H6, into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon (black = C, gray = H).
What are likely formulas for the following substances?
(a) CCl?
(b) AlH?
(c) CH?Cl2
(d) SiF?
(e) CH3NH?
Identify the group of the central element to predict the number of covalent bonds the element can form | ||||||||||||||||||||||
(a) | Carbon (Group 4A) has four electrons in its valence shell and forms four bonds to achieve the noble-gas configuration of neon. A likely formula is CCl4. | |||||||||||||||||||||
| ||||||||||||||||||||||
Write line-bond structures for the following substances, showing all nonbonding electrons:
(a) CHCl3, chloroform
(b) H2S, hydrogen sulfide
(c) CH3NH2, methylamine
(d) CH3Li, methyllithium
Start by drawing the electron-dot structure of the molecule. | ||||||||||||||||||||||
(1) | Determine the number of valence, or outer-shell electrons for each atom in the molecule. For chloroform, we know that carbon has four valence electrons, hydrogen has one valence electron, and each chlorine has seven valence electrons. | |||||||||||||||||||||
(2) | Next, use two electrons for each single bond. | |||||||||||||||||||||
(3) | Finally, use the remaining electrons to achieve a noble gas configuration for all atoms. For a line-bond structure, replace the electron dots between two atoms with a line | |||||||||||||||||||||
| ||||||||||||||||||||||
Why can’t an organic molecule have the formula C2H7?
Each of the two carbons has 4 valence electrons. Two electrons are used to form the carbon–carbon bond, and the 6 electrons that remain can form bonds with a maximum of 6 hydrogens. Thus, the formula C2H7 is not possible.
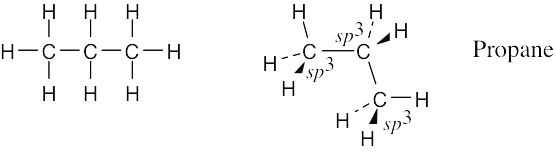
Draw a line-bond structure for propane, CH3CH2CH3. Predict the value of each bond angle, and indicate the overall shape of the molecule.
Connect the carbons and add hydrogens so that all carbons are bonded to four different atoms.
The geometry around all carbon atoms is tetrahedral, and all bond angles are approximately 109°.
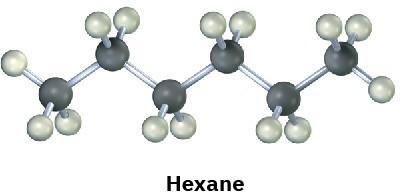
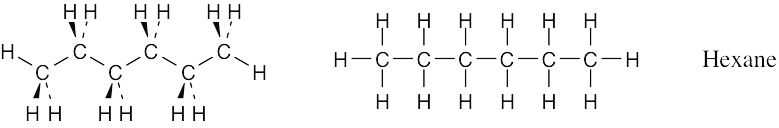
Convert the following molecular model of hexane, a component of gasoline, into a line-bond structure (black = C, gray = H).
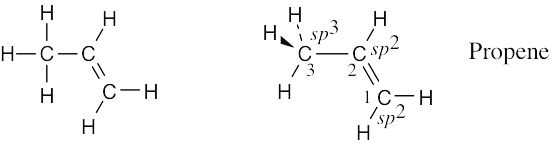
Draw a line-bond structure for propene, CH3CH=CH2. Indicate the hybridization of the orbitals on each carbon, and predict the value of each bond angle.
The C3–H bonds are σ bonds formed by overlap of an sp3 orbital of carbon 3 with an s orbital of hydrogen
The C2–H and C1–H bonds are σ bonds formed by overlap of an sp2 orbital of carbon with an s orbital of hydrogen.
The C2–C3 bond is a σ bond formed by overlap of an sp3 orbital of carbon 3 with an sp2 orbital of carbon 2.
There are two C1–C2 bonds. One is a σ bond formed by overlap of an sp3 orbital of carbon 1 with an sp2 orbital of carbon 2. The other is a π bond formed by overlap of a p orbital of carbon 1 with a p orbital of carbon 2. All four atoms connected to the carbon–carbon double bond lie in the same plane, and all bond angles between these atoms are 120°. The bond angle between hydrogen and the sp3-hybridized carbon is 109°.
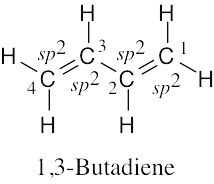
Draw a line-bond structure for 1,3-butadiene, H2C=CH–CH=CH2. Indicate the hybridization of the orbitals on each carbon, and predict the value of each bond angle.
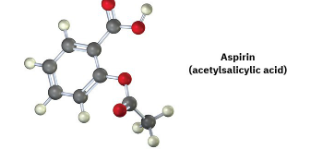
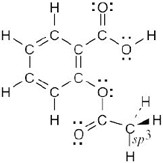
A molecular model of aspirin (acetylsalicylic acid) is shown. Identify the hybridization of the orbitals on each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (black = C, red = O, gray = H).
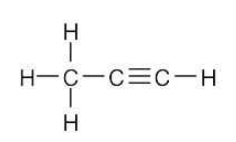
Draw a line-bond structure for propyne, CH3C≡CH. Indicate the hybridization of the orbitals on each carbon, and predict a value for each bond angle.
The C3-H bonds are σ bonds formed by overlap of an sp3 orbital of carbon 3 with an s orbital of hydrogen.
The C1-H bond is a σ bond formed by overlap of an sp orbital of carbon 1 with an s orbital of hydrogen.
The C2-C3 bond is a σ bond formed by overlap of an sp orbital of carbon 2 with an sp3 orbital of carbon 3.
There are three C1-C2 bonds. One is a σ bond formed by overlap of an sp orbital of carbon 1 with an sp orbital of carbon 2. The other two bonds are π bonds formed by overlap of two p orbitals of carbon 1 with two p orbitals of carbon 2.
The three carbon atoms of propyne lie in a straight line: the bond angle is 180°. The H–C1≡C2 bond angle is also 180°. The bond angle between hydrogen and the sp3-hybridized carbon is 109°.
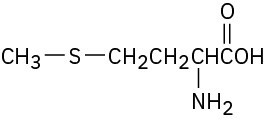
Identify all nonbonding lone pairs of electrons in the following molecules, and tell what geometry you expect for each of the indicated atoms.
(a) The oxygen atom in dimethyl ether, CH3–O–CH3
(b) The nitrogen atom in trimethylamine,
(c) The phosphorus atom in phosphine, PH3
(d) The sulfur atom in the amino acid methionine,
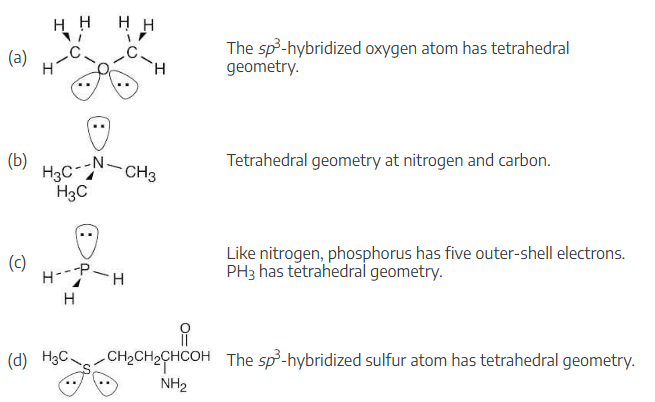
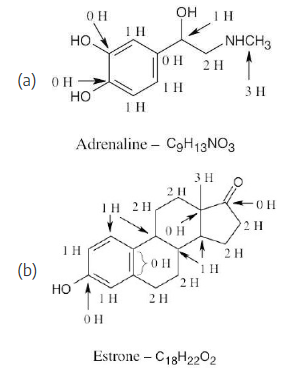
How many hydrogens are bonded to each carbon in the following compounds, and what is the molecular formula of each substance?
Remember that the end of a line represents a carbon atom with 3 hydrogens, a two-way intersection represents a carbon atom with 2 hydrogens, a three-way intersection represents a carbon with 1 hydrogen and a four-way intersection represents a carbon with no hydrogens.
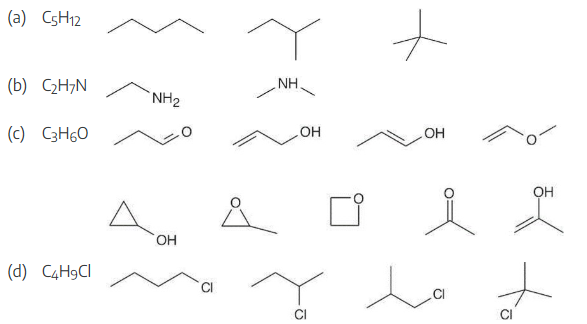
Propose skeletal structures for compounds that satisfy the following molecular formulas: There is more than one possibility in each case.
(a) C5H12
(b) C2H7N
(c) C3H6O
(d) C4H9Cl
Several possible skeletal structures can satisfy each molecular formula.
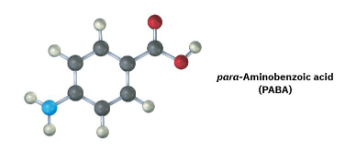
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds, and draw a skeletal structure (black = C, red = O, blue = N, gray = H).
Which element in each of the following pairs is more electronegative?
(a) Li or H
(b) B or Br
(c) Cl or I
(d) C or H
After solving this problem, use Figure 1.20 to check your answers. The larger the number, the more electronegative (EN) the element.
| ||||||||||||||||
Carbon is slightly more electronegative than hydrogen. |
Use the δ+/δ– convention to indicate the direction of expected polarity for each of the bonds indicated.
(a) H3C-Cl
(b) H3C-NH2
(c) H2N-H
(d) H3C-SH
(e) H3C-MgBr
(f) H3C-F
As in Problem 1.19, use Figure 1.20. The partial negative charge is placed on the more electronegative atom, and the partial positive charge is placed on the less electronegative atom.
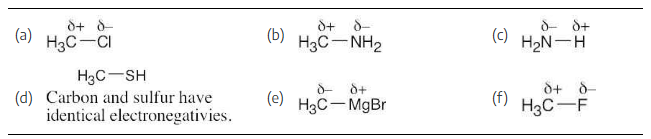
Use the electronegativity values shown in Figure 1.20 to rank the following bonds from least polar to most polar: H3C–Li, H3C–K, H3C–F, H3C–MgBr, H3C–OH
Use Figure 1.20 to find the electronegativities of each element. Calculate ΔEN and rank the answers in order of increasing ΔEN. | |||||||||||||||||||||||||||||||||||||||||||
The most polar bond has the largest ΔEN. Thus, in order of increasing bond polarity: H3C — OH < H3C — MgBr < H3C — Li, H3C — F < H3C — K |
Look at the following electrostatic potential map of methylamine, a substance responsible for the odor of rotting fish, and tell the direction of polarization of the C–N bond:
In an electrostatic potential map, the color red indicates regions of a molecule that are electron-rich. The map shows that nitrogen is the most electronegative atom in chloromethane, and the direction of polarity of the C–N bond is:
Calculate formal charges for the nonhydrogen atoms in the following molecules:
To find the formal charge of an atom in a molecule, follow these two steps:
(1) Draw an electron-dot structure of the molecule.
(2) Use the formula in Section 1.14 (shown below) to determine formal charge for each atom. The periodic table shows the number of valence electrons of the element, and the electron-dot structure shows the number of bonding and nonbonding electrons.
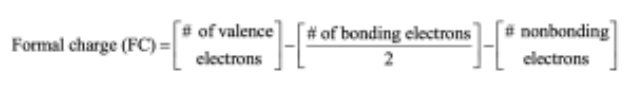
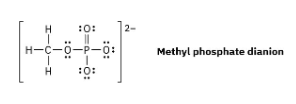
Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in the methyl phosphate dianion.
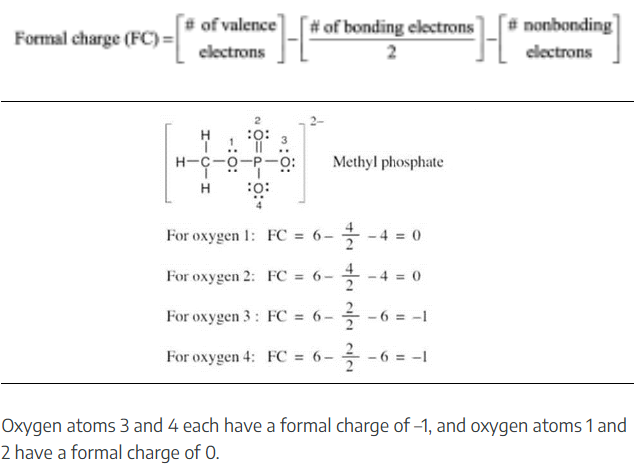
Nitric acid (HNO3) reacts with ammonia (NH3) to yield ammonium nitrate. Write the reaction, and identify the acid, the base, the conjugate acid product, and the conjugate base product.
When an acid loses a proton, the product is the conjugate base of the acid. When a base gains a proton, the product is the conjugate acid of the base.
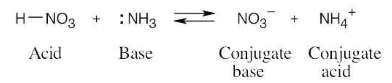
The amino acid phenylalanine has pKa = 1.83, and tryptophan has pKa = 2.83. Which is the stronger acid?
Recall from Section 1.18 that a stronger acid has a smaller pKa and a weaker acid has a larger pKa. Accordingly, phenylalanine (pKa = 1.83) is a stronger acid than tryptophan (pKa = 2.83).
Amide ion, H2N–, is a much stronger base than hydroxide ion, HO–. Which is the stronger acid, NH3 or H2O? Explain.
HO–H is a stronger acid than H2N–H. Since H2N– is a stronger base than HO–, the conjugate acid of H2N– (H2N–H) is a weaker acid than the conjugate acid of HO– (HO–H).

Will either of the following reactions take place to a significant extent as written, according to the data in Table 1.6?
Use Table 1.6 to find the strength of each acid. A reaction takes place as written if the stronger acid is the reactant.
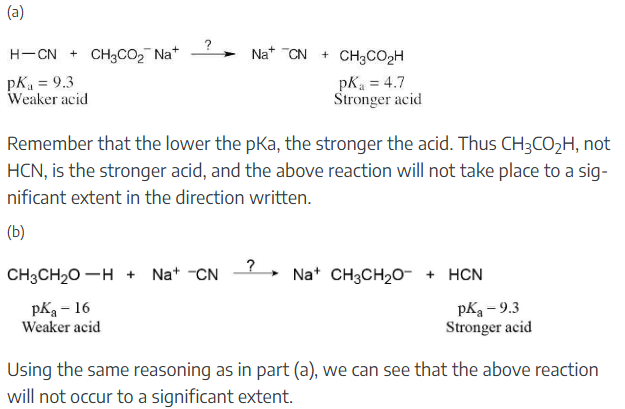
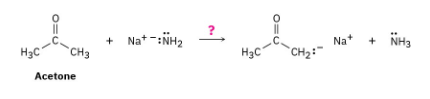
Ammonia, NH3, has pKa ≈ 36, and acetone has pKa ≈ 19. Will the following reaction take place to a significant extent?
As written, the above reaction will take place to virtual completion due to the large difference in pKa values.
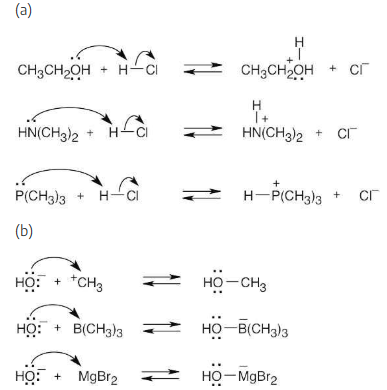
Using curved arrows, show how the species in part (a) can act as Lewis bases in their reactions with HCl, and show how the species in part (b) can act as Lewis acids in their reaction with OH–.
(a) CH3CH2OH, HN(CH3)2, P(CH3)3
(b) H3C+, B(CH3)3, MgBr2
Locate the electron pair(s) of the Lewis base and draw a curved arrow from the electron pair to the Lewis acid. The electron pair moves from the atom at the tail of the arrow (Lewis base) to the atom at the point of the arrow (Lewis acid). (Note: electron dots have been omitted from Cl– to reduce clutter.)
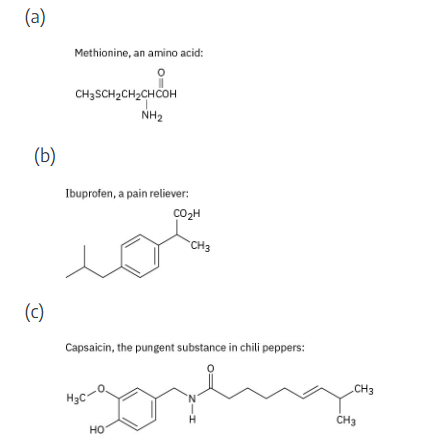
Use Table 2.1 to identify the functional groups in each of the following molecules:
Notice that certain functional groups have different designations if other functional groups are also present in a molecule. For example, a molecule containing a carbon–carbon double bond and no other functional group is an alkene; if other groups are present, the group is referred to as a carbon–carbon double bond. Similarly, a compound containing a benzene ring, and only carbon- and hydrogen-containing substituents, is an arene; if other groups are present, the ring is labeled an aromatic ring.
Propose structures for simple molecules that contain the following functional groups:
(a) Alcohol
(b) Aromatic ring
(c) Carboxylic acid
(d) Amine
(e) Both ketone and amine
(f) Two double bonds
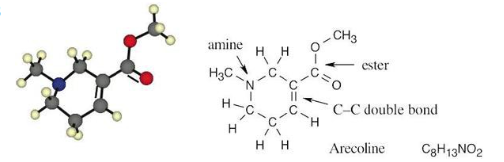
Identify the functional groups in the following model of arecoline, a veterinary drug used to control worms in animals. Convert the drawing into a line-bond structure and a molecular formula (red = O, blue = N, black = C, gray = H).
Draw structures of the five isomers of C6H14.
We know that carbon forms four bonds and hydrogen forms one bond. Thus, draw all possible six-carbon skeletons and add hydrogens so that all carbons have four bonds. To draw all possible skeletons in this problem: (1) Draw the six-carbon straight-chain skeleton; (2) Draw a five-carbon chain, identify the different types of carbon atoms on the chain, and add a –CH3 group to each of the different types of carbons, generating two skeletons; (3) Repeat the process with the four-carbon chain to give rise to the last two skeletons. Add hydrogens to the remaining carbons to complete the structures.
Propose structures that meet the following descriptions:
(a) Two isomeric esters with the formula C5H10O2
(b) Two isomeric nitriles with the formula C4H7N

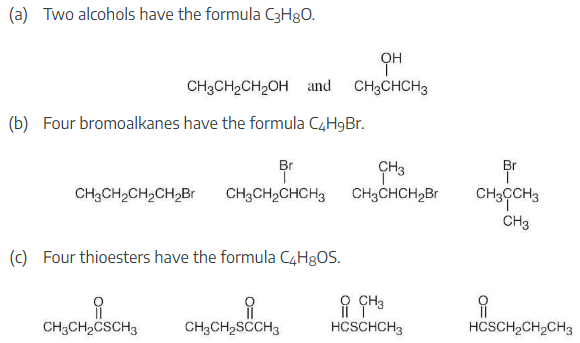
How many isomers are there with the following descriptions?
(a) Alcohols with the formula C3H8O
(b) Bromoalkanes with the formula C4H9Br
(c) Thioesters with the formula C4H8OS
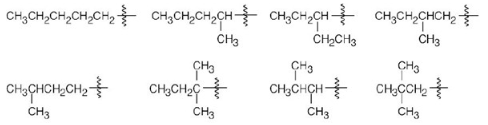
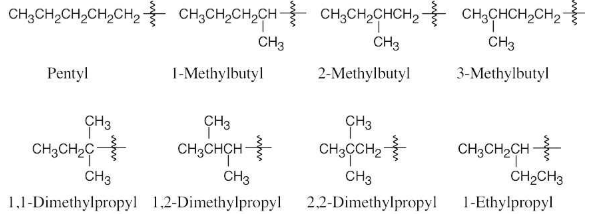
Draw the eight 5-carbon alkyl groups (pentyl isomers).
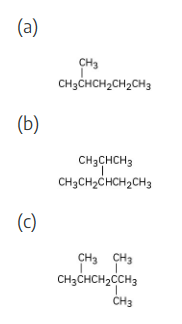
Identify the carbon atoms in the following molecules as primary, secondary, tertiary, or quaternary:
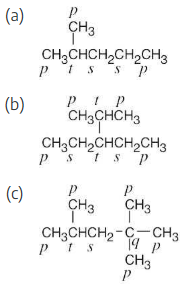
Identify the hydrogen atoms on the compounds shown in Problem 2.8 as primary, secondary, or tertiary.
The carbons and the attached hydrogens have the same classification.
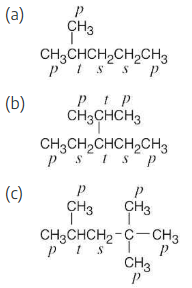
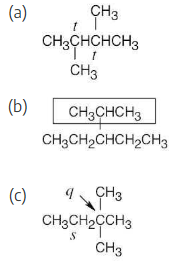
Draw structures of alkanes that meet the following descriptions:
(a) An alkane with two tertiary carbons
(b) An alkane that contains an isopropyl group
(c) An alkane that has one quaternary and one secondary carbon
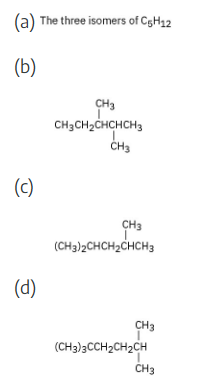
Give IUPAC names for the following compounds:

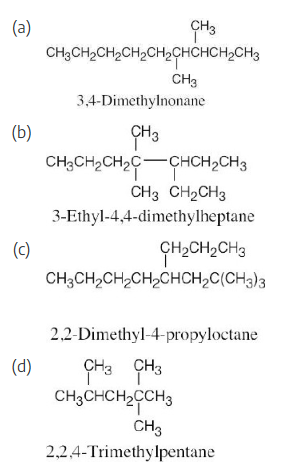
Draw structures corresponding to the following IUPAC names:
(a) 3,4-Dimethylnonane
(b) 3-Ethyl-4,4-dimethylheptane
(c) 2,2-Dimethyl-4-propyloctane
(d) 2,2,4-Trimethylpentane
When you are asked to draw the structure corresponding to a given name, draw the parent carbon chain, attach the specified groups to the proper carbons, and fill in the remaining hydrogens.
Name the eight 5-carbon alkyl groups you drew in Problem 2.7.
Give the IUPAC name for the following hydrocarbon, and convert the drawing into a skeletal structure.
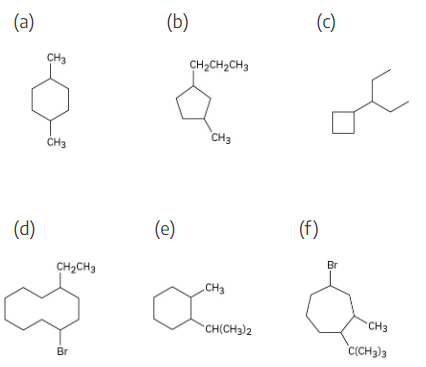
Give IUPAC names for the following cycloalkanes:
The steps for naming a cycloalkane are very similar to the steps used for naming an open- chain alkane.
Step 1: Name the parent cycloalkane. In (a), the parent is cyclohexane. If the compound has an alkyl substituent with more carbons than the ring size, the compound is named as a cycloalkyl-substituted alkane, as in (c).
Step 2: Identify the substituents. In (a), both substituents are methyl groups.
Step 3: Number the substituents so that the second substituent receives the lowest possible number. In (a), the substituents are in the 1- and 4- positions.
Step 4: Name the compound. If two different alkyl groups are present, cite them alphabetically. Halogen substituents follow the same rules as alkyl substituents.
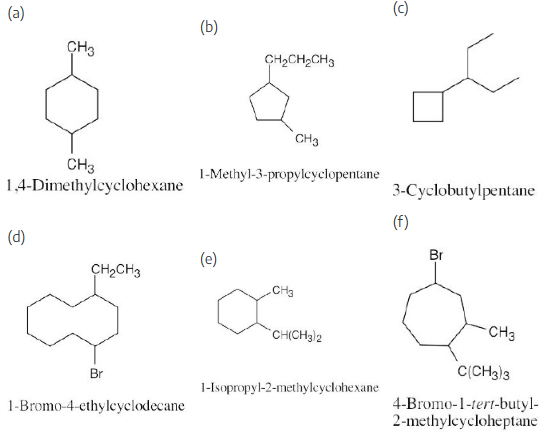
Draw structures corresponding to the following IUPAC names:
(a) 1,1-Dimethylcyclooctane
(b) 3-Cyclobutylhexane
(c) 1,2-Dichlorocyclopentane
(d) 1.3-Dibromo-5-methylcyclohexane
To draw a substituted cycloalkane, simply draw the ring and attach substituents in the specified positions. The structure in (b) is named as a cyclobutyl-substituted alkane because the alkyl chain has more carbons than the ring.
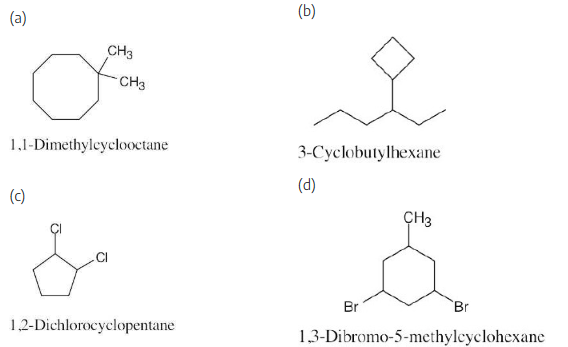
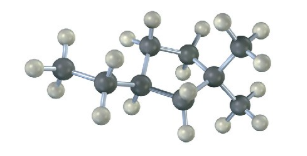
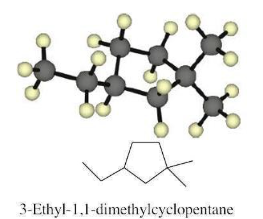
Name the following cycloalkane:
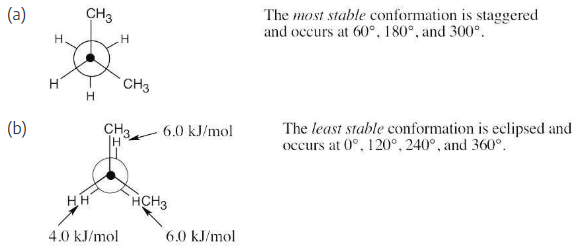
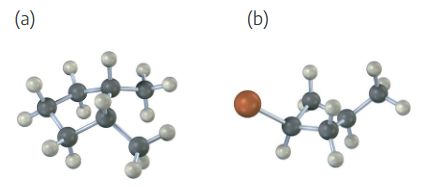
Sight along the C2–C1 bond of 2-methylpropane (isobutane).
(a) Draw a Newman projection of the most stable conformation.
(b) Draw a Newman projection of the least stable conformation.
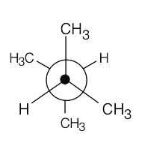
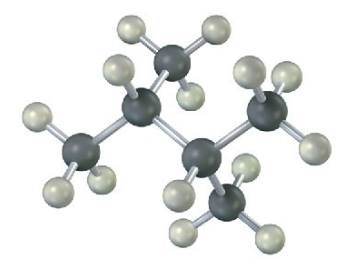
Sight along the C2–C3 bond of 2,3-dimethylbutane, and draw a Newman projection of the most stable conformation.
This conformation of 2,3-dimethylbutane is the most stable because it is staggered and has the fewest CH3↔CH3 gauche interactions.
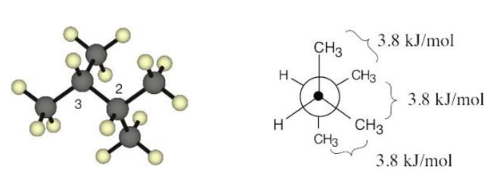
Draw a Newman projection along the C2–C3 bond of the following conformation of 2,3- dimethylbutane.
The conformation is a staggered conformation in which the hydrogens on carbons 2 and 3 are 60° apart. Draw the Newman projection.
The Newman projection shows three gauche interactions, each of which has an energy cost of 3.8 kJ/mol. The total strain energy is 11.4 kJ/mol (3 × 3.8 kJ/mol).
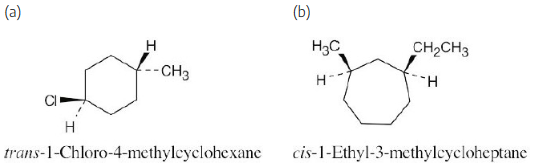
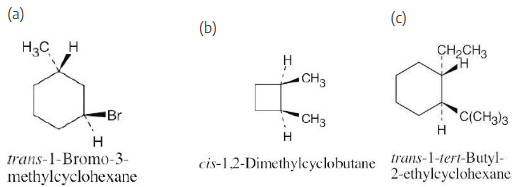
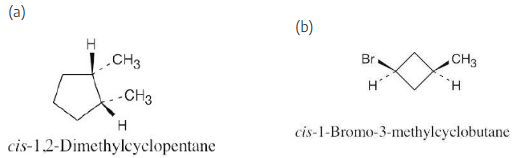
Name the following substances, including the cis– or trans– prefix:
Two substituents are cis if they both have either dashed or wedged bonds. The substituents are trans if one has a wedged bond and the other has a dashed bond.
Draw the structures of the following molecules:
(a) trans-1-Bromo-3-methylcyclohexane
(b) cis-1,2-Dimethylcyclobutane
(c)trans-1-tert-Butyl-2-ethylcyclohexane
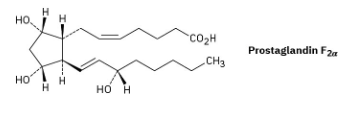
Prostaglandin F2α, a hormone that causes uterine contraction during childbirth, has the following structure. Are the two hydroxyl groups (–OH) on the cyclopentane ring cis or trans to each other? What about the two carbon chains attached to the ring?
The two hydroxyl groups on the cyclopentyl ring are cis because they both point behind the plane of the page (both dashed bonds). The carbon chains have a trans relationship (one is dashed and the other is wedged).
Name the following substances, including the cis– or trans– prefix (red-brown = Br):
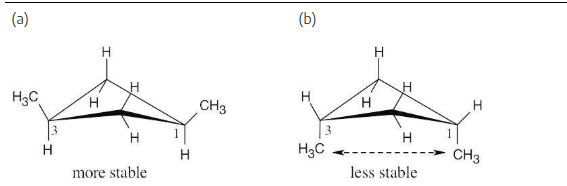
cis-1,2-Dimethylcyclopropane has more strain than trans-1,2-dimethylcyclopropane. How can you account for this difference? Which of the two compounds is more stable?
The added energy cost of eclipsing interactions causes cis-1,2-dimethylcyclopropane to be of higher energy and to be less stable than the trans isomer.

Two conformations of cis-1,3-dimethylcyclobutane are shown. What is the difference between them, and which do you think is likely to be more stable?
The methyl groups are farther apart in the more stable conformation of cis-1,3- dimethylcyclobutane.
Draw two different chair conformations of cyclohexanol (hydroxycyclohexane), showing all hydrogen atoms. Identify each position as axial or equatorial.
Use the technique in Section 2.12 to draw the cyclohexane ring. Figure 2.12 shows how to attach axial (a) and equatorial (e) bonds.
The conformation with –OH in the equatorial position is more stable.
Note: The starred ring carbons lie in the plane of the paper.

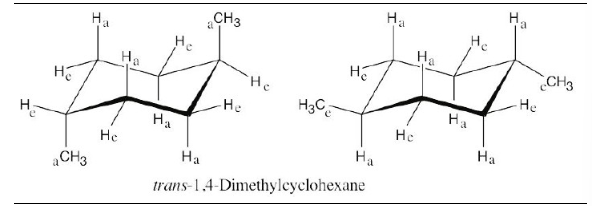
Draw two different chair conformations of trans-1,4-dimethylcyclohexane, and label all positions as axial or equatorial.
In trans-1,4-disubstituted cyclohexanes, the methyl substituents are either both axial or both equatorial.
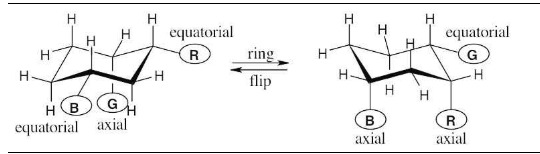
Identify each of the colored positions—red, blue, and green—as axial or equatorial. Then carry out a ring-flip, and show the new positions occupied by each color.
In a ring-flip, an axial substituent becomes equatorial, and an equatorial substituent becomes axial.
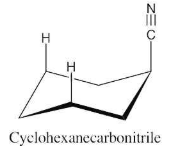
Why do you suppose an axial cyano (–CN) substituent causes practically no 1,3-diaxial steric strain (0.4 kJ/mol)?
There is very little energy difference between an axial and an equatorial cyano group because the small linear cyano group takes up very little room and produces practically no 1,3-diaxial interactions.
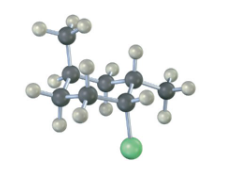
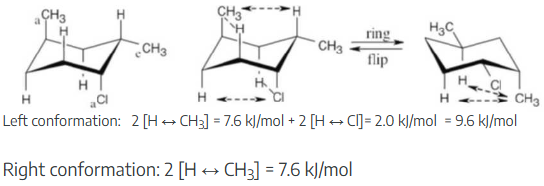
Identify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):
The three substituents have the orientations shown in the first structure. To decide if the conformation shown is the more stable conformation or the less stable conformation, perform a ring-flip on the illustrated conformation and do a calculation of the total strain in each structure as in the previous problem. Notice that each conformation has a Cl – CH3 gauche interaction, but we don’t need to know its energy cost because it is present in both conformations.
The illustrated conformation on the left is the less stable chair form.
Which of the following objects are chiral?
(a) Soda can
(b) Screwdriver
(c) Screw
(d) Shoe
Objects having a plane of symmetry are achiral.
Chiral: screw, shoe.
Achiral: soda can, screwdriver.
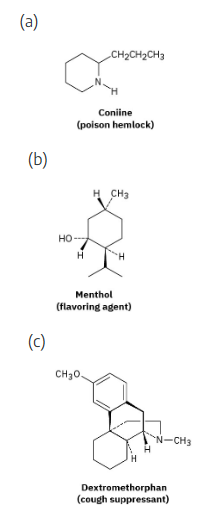
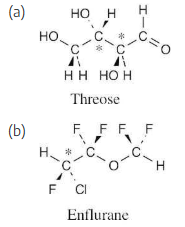
Which of the following molecules are chiral? Identify the chirality center(s) in each.
Use the following rules to locate centers that are not chirality centers, then examine the remaining centers to find a carbon with four different groups attached. | ||
1. | All –CH3 and –CX3 carbons are not chirality centers. | |
2. | All –CH2– and –CX2– carbons are not chirality centers. | |
3. | All 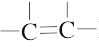 and 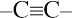 carbons are not chirality centers. By rule 3, all aromatic ring carbons are not chirality centers. | |
(a) | 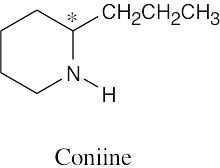 | |
(b) | 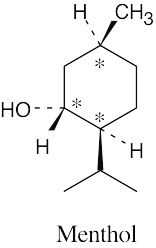 | |
(c) | 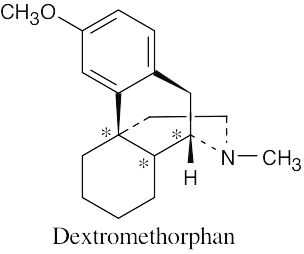 | |
Alanine, an amino acid found in proteins, is chiral. Draw the two enantiomers of alanine using the standard convention of solid, wedged, and dashed lines.
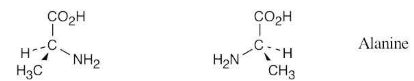

Identify the chirality centers in the following molecules (gray = H, black = C, red = O, green = Cl, yellow-green = F):
Is cocaine (Worked Example 3.2) dextrorotatory or levorotatory?
By convention, a (–) rotation indicates rotation to the left, and thus cocaine is levorotatory.
Which member in each of the following sets ranks higher?
(a)−H or −Br
(b) −Cl or −Br
(c) -CH3 or -CH2CH3
(d) −NH2 or −OH
(e) −CH2OH or −CH3
(f) −CH2OH or −CH=O
Review the sequence rules presented in Section 3.5. A summary: | ||||||||||||||||||||||||||||||
Rule 1: | An atom with a higher atomic number has priority over an atom with a lower atomic number. | |||||||||||||||||||||||||||||
Rule 2: | If a decision can’t be reached by using Rule 1, look at the second, third, or fourth atom away from the double-bond carbon until a decision can be made. | |||||||||||||||||||||||||||||
Rule 3: | Multiple-bonded atoms are equivalent to the same number of single-bonded atoms. | |||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
Rank each of the following sets of substituents:
(a) −H, −OH, −CH2CH3, −CH2CH2OH
(b) −CO2H, −CO2CH3, −CH2OH, −OH
(c) −CN, −CH2NH2, −CH2NHCH3, −NH2
(d) −SH, −CH2SCH3, −CH3, −SSCH3
Use the sequence rules in Section 3.5.
(a) By Rule 1, –H is of lowest priority, and –OH is of highest priority. By Rule 2, –CH2CH2OH is of higher priority than –CH2CH3.
Highest → Lowest
–OH, –CH2CH2OH, –CH2CH3, –H
(b) By Rule 3, –CO2H is considered as. Because 3 oxygens are attached to a –CO2H carbon and only one oxygen is attached to –CH2OH, –CO2H is of higher priority than -CH2OH. –CO2CH3 is of higher priority than –CO2H by Rule 2, and –OH is of highest priority by Rule 1.
Highest → Lowest
(b) –OH, –CO2CH3, –CO2H, –CH2OH
(c) –NH2, –CN, –CH2NHCH3, –CH2NH2
(d) –SSCH3, –SH, –CH2SCH3, –CH3
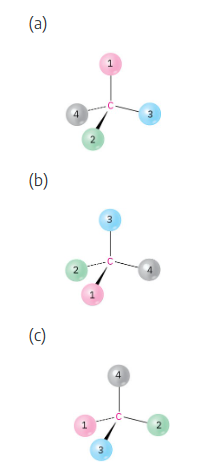
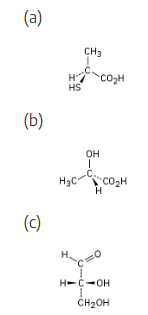
Orient each of the following drawings so that the lowest-ranked group is toward the rear, and then assign R or S configuration:
All stereochemistry problems are easier if you use models. Part (a) will be solved by two methods – with models and without models. | ||
(a) | With models: Build a model of (a). Orient the model so that group 4 is pointing to the rear. Note the direction of rotation of arrows that go from group 1 to group 2 to group. The arrows point counterclockwise, and the configuration is S. Without models: Imagine yourself looking at the molecule, with the group of lowest priority pointing to the back. Your viewpoint would be at the upper right of the molecule, and you would see group 1 on the left, group 3 on the right and group 2 at the bottom. The arrow of rotation travels counterclockwise, and the configuration is S. | |
(a) | 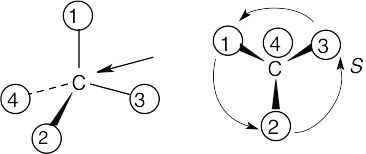 | |
(b) | 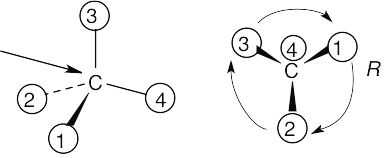 | |
(c) | 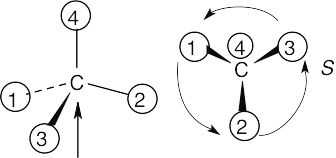 | |
Assign R or S configuration to the chirality center in each of the following molecules:
Step 1: | For each chirality center, rank substituents by the Cahn–Ingold–Prelog system; give the number 4 to the lowest priority substituent. For part (a):
| ||||||||||||
Step 2: | As in the previous problem, orient yourself so that you are 180° from the lowest priority group (indicated by the arrow in the drawing). From that viewpoint, draw the molecule as it looks when you face it. Draw the arrow that travels from group 1 to group 2 to group 3, and note its direction of rotation. The molecule in (a) has S configuration. | ||||||||||||
(a) | 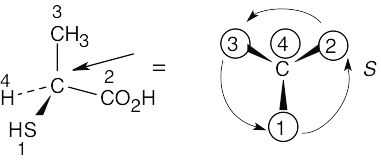 | ||||||||||||
(b) | 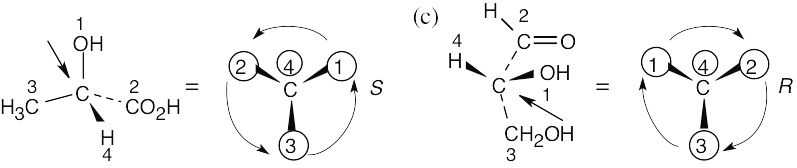 In (b), the observer is behind the page, looking out and down toward the right. In (c), the observer is behind the page looking out and up to the left. | ||||||||||||
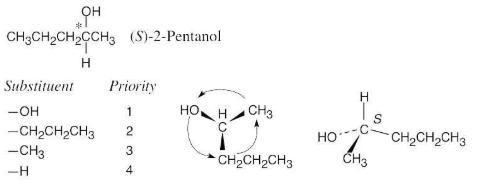
Draw a tetrahedral representation of (S)-2-pentanol (2-hydroxypentane).
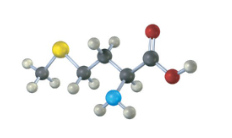
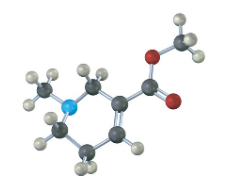
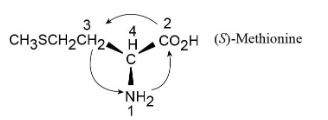
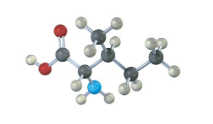
Assign R or S configuration to the chirality center in the following molecular model of the amino acid methionine (blue = N, yellow = S):
Fortunately, methionine is shown in the correct orientation.
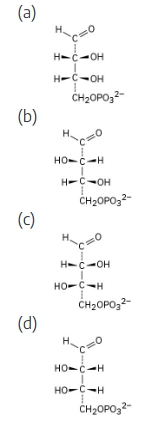
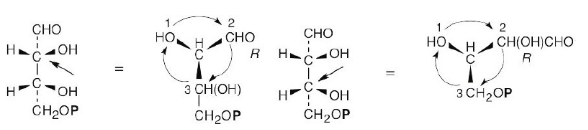
One of the following molecules (a)–(d) is D-erythrose 4-phosphate, an intermediate in the Calvin photosynthetic cycle by which plants incorporate CO2 into carbohydrates. If D-erythrose 4-phosphate has R stereochemistry at both chirality centers, which of the structures is it? Which of the remaining three structures is the enantiomer of D-erythrose 4-phosphate, and which are diastereomers?
For (a): (Note: the phosphate group is represented as P.)
(note attached picture)
(a) R,R (b) S,R (c) R,S (d) S,S
a, d are enantiomers and are diastereomeric with b, c. b, c are enantiomers and are diastereomeric with a, d.
Structure (a) is D-erythrose 4-phosphate, structure (d) is its enantiomer, and structures (b) and (c) are its diastereomers.
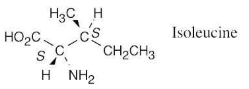
Assign R or S configuration to each chirality center in the following molecular model of the amino acid isoleucine (blue = N):
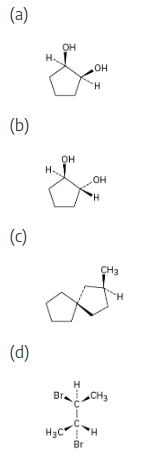
Which of the following structures represent meso compounds?
To decide if a structure represents a meso compound, try to locate a plane of symmetry that divides the molecule into two halves that are mirror images. Molecular models are always helpful. | ||
(a) | 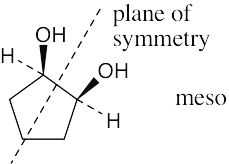 | |
(b) | and (c) are not meso structures | |
(d) | 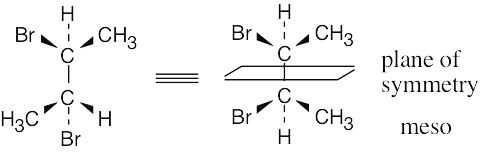 | |
Which of the following have a meso form? (Recall that the –ol suffix refers to an alcohol, ROH.)
(a) 2,3-Butanediol
(b) 2,3-Pentanediol
(c) 2,4-Pentanediol
For a molecule to exist as a meso form, it must possess a plane of symmetry. 2,3- Butanediol can exist as a pair of enantiomers or as a meso compound, depending on the configurations at carbons 2 and 3. | ||
(a) | 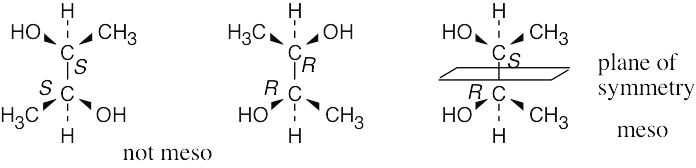 | |
(b) | 2,3-Pentanediol has no symmetry plane and thus can’t exist in a meso form. | |
(c) | 2,4-Pentanediol can exist in a meso form. 2,4-Pentanediol can also exist as a pair of enantiomers (2R,4R) and (2S,4S) that are not meso compounds. | |
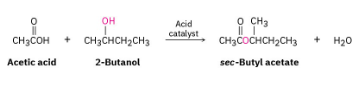
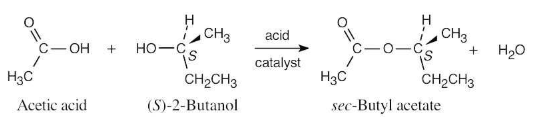
Suppose that acetic acid (CH3CO2H) reacts with (S)-2-butanol to form an ester (see Worked Example 3.6). What stereochemistry would you expect the product(s) to have? What is the relationship of the products?
The product is the pure S-ester. No new chirality centers are formed during the reaction, and the configuration at the chirality center of (S)-2-butanol is unchanged.
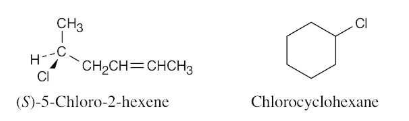
What kinds of isomers are the following pairs?
(a) (S)-5-Chloro-2-hexene and chlorocyclohexane
(b) (2R,3R)-Dibromopentane and (2S,3R)-dibromopentane
(a)
These two compounds are constitutional isomers (skeletal isomers).
(note image)
(b) The two dibromopentane stereoisomers are diastereomers.
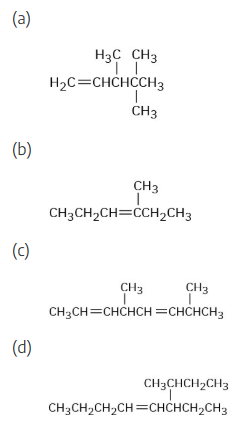
Give IUPAC names for the following compounds:

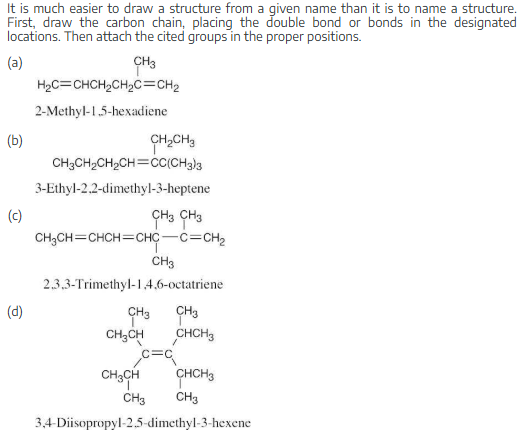
Draw structures corresponding to the following IUPAC names:
(a) Methyl-1,5-hexadiene
(b) Ethyl-2,2-dimethyl-3-heptene
(c) 2,3,3-Trimethyl-1,4,6-octatriene
(d) 3,4-Diisopropyl-2,5-dimethyl-3-hexene
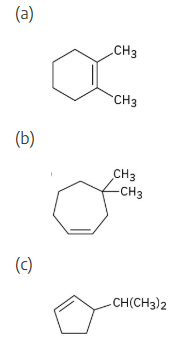
Name the following cycloalkenes:
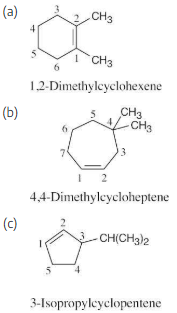
Change the following old names to new, post-1993 names, and draw the structure of each compound:
(a) 2,5,5-Trimethyl-2-hexene
(b) 2,3-Dimethyl-1,3-cyclohexadiene
Alkyne nomenclature follows the general rules for hydrocarbons discussed in Section 2.4 and 4.1. The suffix -yne is used, and the position of the triple bond is indicated by giving the number of the first alkyne carbon in the chain. Numbering the main chain begins at the end nearer the triple bond so that the triple bond receives as low a number as possible.
Compounds with more than one triple bond are called diynes, triynes, and so forth; compounds containing both double and triple bonds are called enynes (not ynenes). Numbering of an enyne chain starts from the end nearer the first multiple bond, whether double or triple. When there is a choice in numbering, double bonds receive lower numbers than triple bonds. For example:
As with alkyl and alkenyl substituents derived from alkanes and alkenes, respectively, alkynyl groups are also possible.
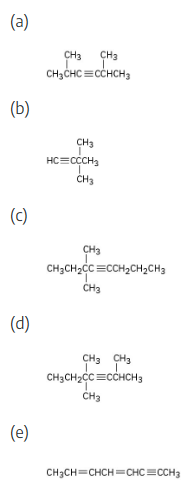
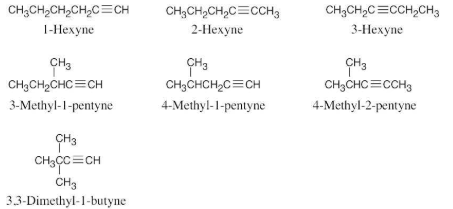
Name the following alkynes:

There are seven isomeric alkynes with the formula C6H10. Draw and name them.
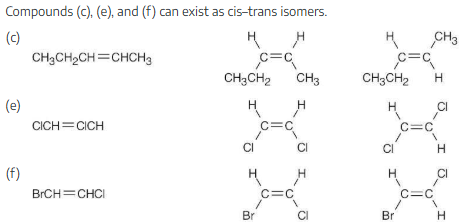
Which of the following compounds can exist as pairs of cis–trans isomers? Draw each pair, and indicate the geometry of each isomer.
(a) CH3CH=CH2
(b) (CH3)2C=CHCH3
(c) CH3CH2CH=CHCH3
(d)(CH3)2C=C(CH3)CH2CH3
(e) ClCH=CHCl
(f) BrCH=CHCl
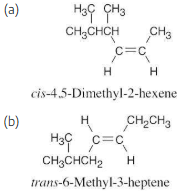
Name the following alkenes, including a cis or trans designation:
Which member in each of the following sets ranks higher?
(a)−H or −CH3
(b)−Cl or −CH2Cl
(c)–CH2CH2Br or –CH=CH2
(d)−NHCH3 or −OCH3
(e)−CH2OH or −CH=O
(f)−CH2OCH3 or −CH=O
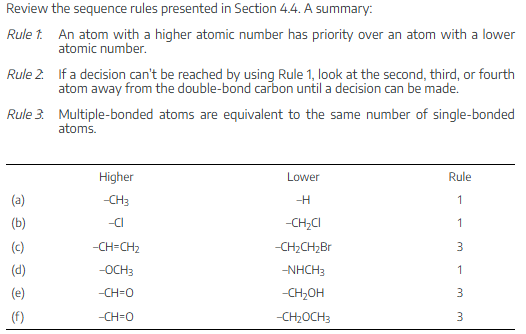
Rank the substituents in each of the following sets according to the sequence rules:
(a)−CH3, −OH, −H, −Cl
(b)−CH3, −CH2CH3, −CH=CH2, −CH2OH
(c)−CO2H, −CH2OH, −C≡N, −CH2NH2
(d)−CH2CH3, −C≡CH, −C≡N, −CH2OCH3
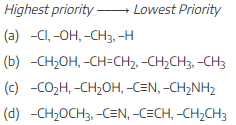
Assign E or Z configuration to the following alkenes:
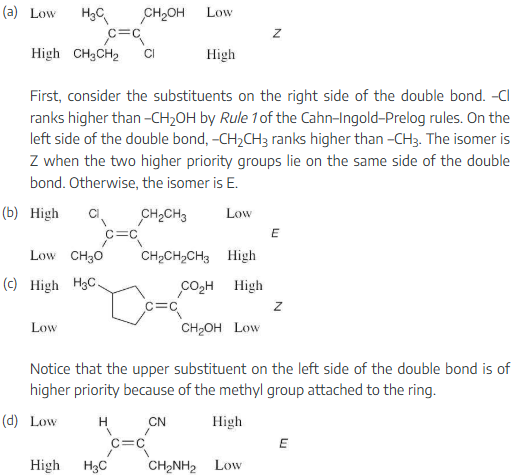
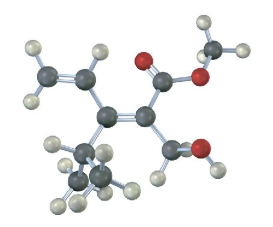
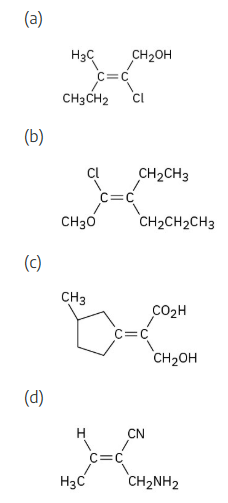
Assign stereochemistry (E or Z) to the double bond in the following compound, and convert the drawing into a skeletal structure (red = O):
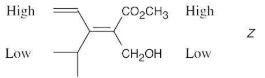
Classify each of the following reactions as an addition, elimination, substitution, or rearrangement:
(a) CH3Br + KOH ⟶ CH3OH + KBr
(b) CH3CH2Br ⟶ H2C=CH2 + HBr
(c) H2C=CH2 + H2 ⟶ CH3CH3
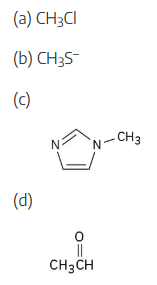
Which of the following species are likely to be nucleophiles and which electrophiles? Which might be both?
Keep in mind:
(1) An electrophile is electron-poor, either because it is positively charged, because it has a functional group that is positively polarized, or because it has a vacant orbital. |
(2) A nucleophile is electron-rich, either because it has a negative charge, because it has a functional group containing a lone electron pair, or because it has a functional group that is negatively polarized. |
(3) Some molecules can act as both nucleophiles and electrophiles, depending on the reaction conditions. (a) The electron-poor carbon acts as an electrophile. (b) CH3S– is a nucleophile because of the sulfur lone-pair electrons and because it is negatively charged. (c) C4H6N2 is a nucleophile because of the lone-pair electrons of nitrogen. (Only one of the nitrogens is nucleophilic, for reasons that will be explained in a later chapter.) (d) CH3CHO is both a nucleophile and an electrophile because of its polar C=O bond. |
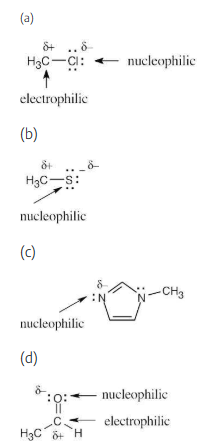
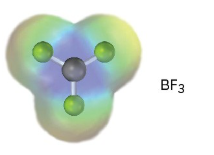
An electrostatic potential map of boron trifluoride is shown. Is BF3 likely to be a nucleophile or an electrophile? Draw a Lewis structure for BF3, and explain your answer.
BF3 is likely to be an electrophile because the electrostatic potential map indicates that it is electron-poor (blue). The electron-dot structure shows that BF3 lacks a complete electron octet and can accept an electron pair from a nucleophile.

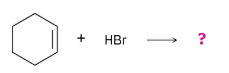
What product would you expect from reaction of cyclohexene with HBr? With HCl?
Reaction of cyclohexene with HCl or HBr is an electrophilic addition reaction in which halogen acid adds to a double bond to produce a haloalkane.
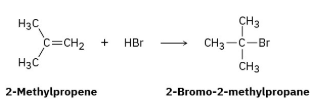
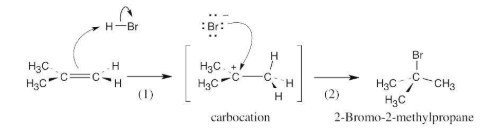
Reaction of HBr with 2-methylpropene yields 2-bromo-2-methylpropane. What is the structure of the carbocation formed during the reaction? Show the mechanism of the reaction.
The mechanism is pictured in Figure 4.7.
The steps:
(1) Attack of the π electrons of the double bond on HBr, forming a carbocation
(2) Formation of a C–Br bond by electron pair donation from Br– to form the neutral addition product
Which reaction is faster, one with ΔG‡ = +45 kJ/mol or one with ΔG‡ = +70 kJ/mol?
A reaction with ΔG‡ = 45 kJ/mol is faster than a reaction with ΔG‡ = 70 kJ/mol because a larger value for ΔG‡ indicates a slower reaction.
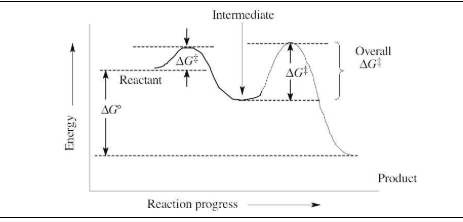
Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall ΔG‡, and overall ΔG°.
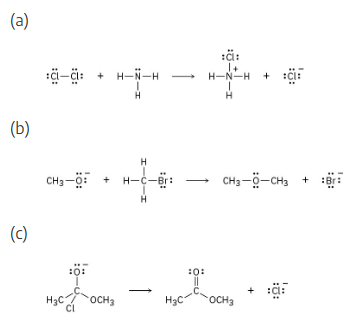
Add curved arrows to the following polar reactions to indicate the flow of electrons in each:
For curved arrow problems, follow these steps:
(1) Locate the bonding changes. In (a), a bond from nitrogen to chlorine has formed, and a Cl-Cl bond has broken.
(2) Identify the nucleophile and electrophile (in (a), the nucleophile is ammonia and the electrophile is one Cl in the Cl2 molecule), and draw a curved arrow whose tail is near the nucleophile and whose head is near the electrophile.
(3) Check to see that all bonding changes are accounted for. In (a), we must draw a second arrow to show the unsymmetrical bond-breaking of Cl2 to form Cl–.
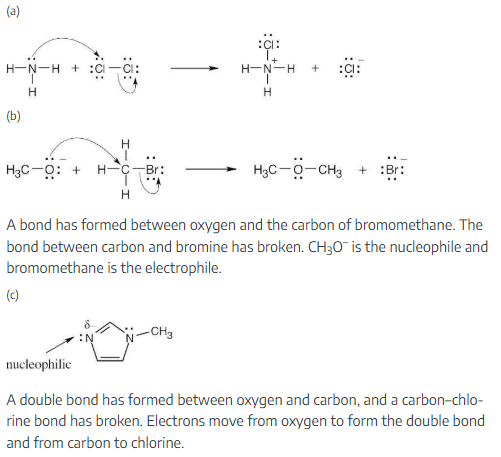
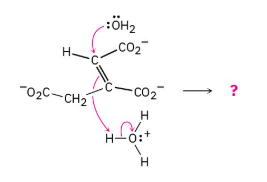
Predict the products of the following polar reaction, a step in the citric acid cycle for food metabolism, by interpreting the flow of electrons indicated by the curved arrows:
This mechanism will be studied in a later chapter.
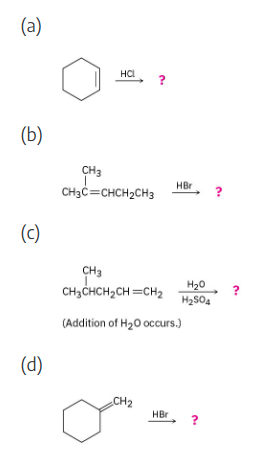
Predict the products of the following reactions:
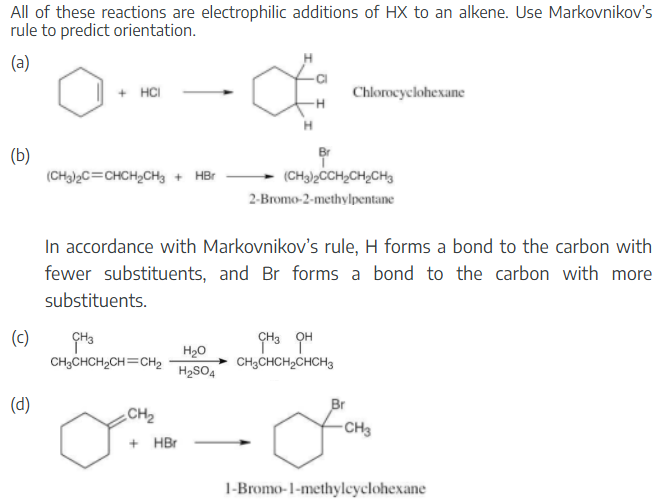
All of these reactions are electrophilic additions of HX to an alkene. Use Markovnikov’s rule to predict orientation.
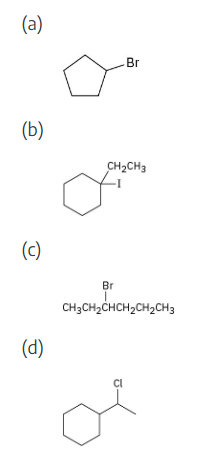
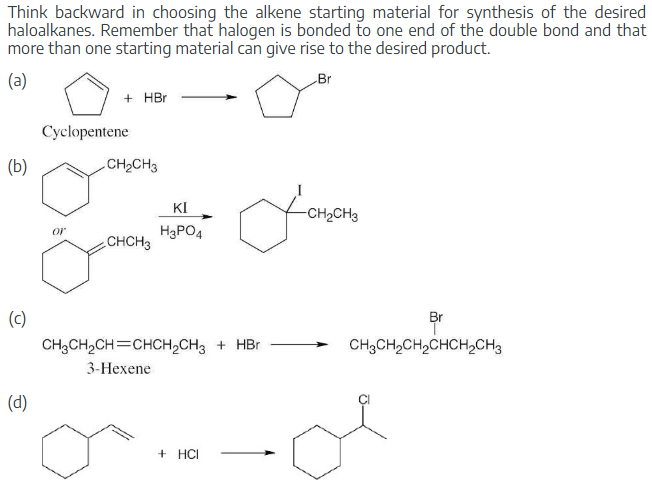
What alkenes would you start with to prepare the following products?
Think backward in choosing the alkene starting material for synthesis of the desired haloalkanes. Remember that halogen is bonded to one end of the double bond and that more than one starting material can give rise to the desired product.