Nucleophilic Substitution
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
26 Terms
What can be said about the covalent bond between the carbon and halogen atom in a halogenoalkane?
The covalent bond between the carbon and halogen atom is highly polar.
Chlorine is δ- and the carbon that is attached to the chorine is δ+.
Why is the covalent bond between the carbon and halogen atom polar?
Halogen atoms are more electronegative than carbon atoms and will therefore attract the pair of electrons in the covalent bond more strongly than carbon.
This produces a permanent dipole-dipole force. The ∂+ carbon is susceptible to nucleophilic attack which leads to substitution reactions.
What is electronegativity?
The ability of an atom to attract electron density towards itself within a covalent bond.
What happens to the electronegativity as you move down a group?
Electronegativity decreases.
This means fluorine is the most electronegative halogen, and iodine is the least electronegative halogen.
Carbon-fluorine bonds are the most polar, and carbon-iodine are the least polar.
Why do electronegativity decrease as you move down a group?
Electronegativity decreases as the size of the halogen increases.
What is a nucleophile?
A nucleophile is a species that has a lone pair of electrons that can be donated to an electron-deficient species.
Nucleophiles are usually negatively charged.
Explain why halogenoalkanes are able to attract nucleophiles.
The halogen in a carbon-halogen bond has a partial negative charge, and the carbon atom has a partial positive charge.
This partially positive carbon atom attracts electron donating species (nucleophiles).
Describe the nucleophilic substitution reaction for a halogenoalkane (chloromethane) and a hydroxide ion (OH-).
The nucleophile is the hydroxide ion, OH-; which is provided by aqueous sodium hydroxide. It has lone pairs on the oxygen atom, so is therefore a good nucleophile.
The lone pair on the hydroxide ion is donated to the δ+ carbon in the chloromethane, following attack by the nucleophile on the positive atom.
Because the lone pair is donated, a bond is formed
between the OH- and the carbon. The carbon-chlorine bond breaks to release the chloride ion. The product is methanol.
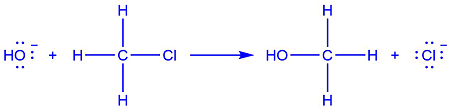
Describe one other way in which the nucleophilic substitution reaction of a halogenoalkane can occur.
The same reaction can be achieved using OH- in water ∴ this reaction can also be called a hydrolysis reaction.
What is a hydrolysis reaction?
A reaction in which water breaks a bond to form a new product.
Diagram showing the nucleophilic substitution reaction of bromoethane a Nu- ion.
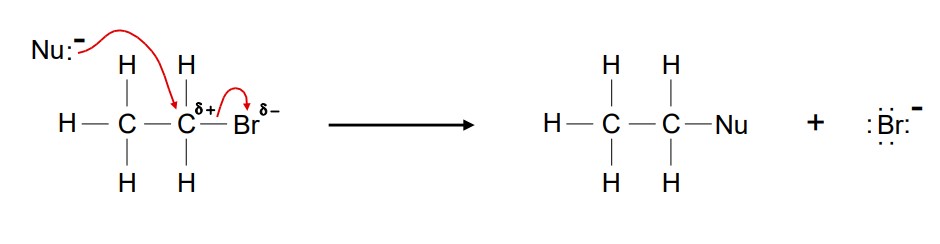
State the conditions needed to carry out a nucleophilic substitution reaction of a halogenoalkane to form an alcohol.
This process is carried out under *reflux in aqueous solution.
*The process of continuous evaporation and condensation of reactions.
What does the reactivity of halogenoalkanes depend on?
The reactivity of halogenoalkanes depends on how easily the C-X bond breaks.
State the order in which halogenoalkanes react, from most reactive to less reactive.
Iodo > Bromo > Chloro
Which factor is more important in predicting the rate of reaction: bond polarity or bond strength / enthalpy?
Bond strength.
Explain why bond enthalpy decreases as you go down the halogen group.
Bond enthalpy is the energy required to break a bond.
The positive nucleus of the halogen is further away from the negative pair of electrons as you go down the group; the electrostatic attraction is weaker, so there is a weaker covalent bond.
Therefore, we expect C-F bond to be the least reactive and C-I to be the most reactive.
Explain why the C-Cl bond is the strongest carbon-halogen bond and, therefore, is the hardest to break.
Substitution involves breaking the carbon-halogen bond.
The C-Cl bond is the strongest because chlorine is the smallest halogen and can get closer to the carbon atom.
This means the negative electrons of the chlorine can be more strongly attracted to the positive nucleus of the carbon.
Therefore, the C-Cl bond is the hardest bond to break.
How can the rate of hydrolysis be measured?
By recording the rate at which the halide ions, X-, form a precipitate (AgX) with Ag+ ions.
This is the test for halogens.
State the test for halogens.
The first part of the process is hydrolysis, where aqueous sodium hydroxide (NaOH) is added to the organic compound and heated.
RX+ NaOH(aq) → ROH + Na+(aq) + X- (aq)
Add dilute nitric acid (HNO₃) to neutralise the excess sodium hydroxide (this is to stop it from interfering with the test).
Then add aqueous silver nitrate, AgNO3 (aq), to detect the presence of the halide ion.
This will yield a silver halide precipitate as shown by the ionic equation:
X- + Ag+ → AgX (s)
where X = the halide.
What colour precipitate does a chloride ion produced when reacted with silver nitrate?
White.
What colour precipitate does a bromide ion produced when reacted with silver nitrate?
Cream.
What colour precipitate does a iodide ion produced when reacted with silver nitrate?
Yellow.
Why must further testing with ammonia (NH3) be carried out in order to detect the halide ion present?
The colour of the precipitate formed can be unclear- ammonia can be used to distinguish it.
What happens to the precipitate containing chloride ions when ammonia is added?
Dissolves in dilute ammonia.
What happens to the precipitate containing bromide ions when ammonia is added?
Dissolves in concentrated ammonia.
What happens to the precipitate containing iodide ions when ammonia is added?
Does NOT dissolve (in either dilute or concentrated ammonia).