Chemistry - Chapter 6 Enthalpy Changes
1/25
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
The 2 different types of reactions, and their definitions and properties.
Exothermic reactions
A reaction in which heat is released to the surroundings. ∆H is negative.
The temperature of surroundings increase.
Combustion, neutralisation and oxidation (respiration) are exothermic reactions.
Endothermic reactions
A reaction in which heat is absorbed from the surroundings. ∆H is positive.
The temperature of surroundings decreases.
Decomposition, photosyntheses, dissolving of some ammonium salts. is an endothermic reaction.
What do the surroundings include?
Reaction mixture in test-tube
Test tube itself
Air surrounding test tube
Anything dipping inside reaction mixture.
Define enthalpy change.
Give an equation representing ∆H and state the units.
The heat energy transferred during a chemical reaction.
Equation:
∆H = Hproducts - Hreactants
Units: kJmol-1
Define reaction pathway diagrams.
Shows the relative enthalpies of the reactants (on left) and products (on right) and the ∆H as an arrow. It may also show activation energy.
Define activation energy.
The minimum amount of energy colliding particles must possess in order to break bonds and start a chemical reaction.
The ∆H of Ea is always a +ve value, because enough energy must be absorbed to increase the KE of molecules in order for them to collide with enough energy to break bonds.
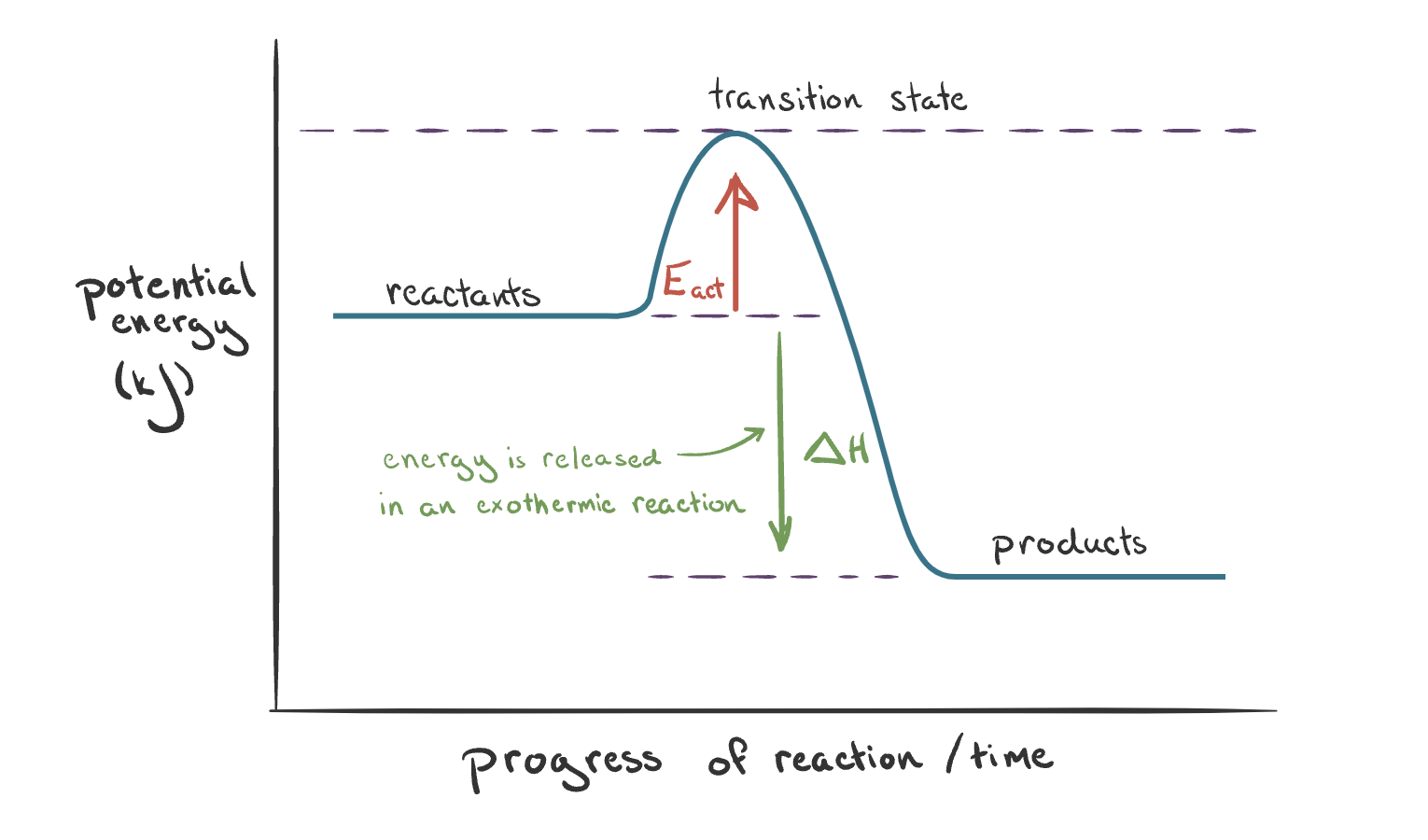
The reaction pathway diagram for an exothermic reaction. Explain.
Energy released to surroundings:
The enthalpy of reactants > products.
∆H is negative (arrow points downwards).
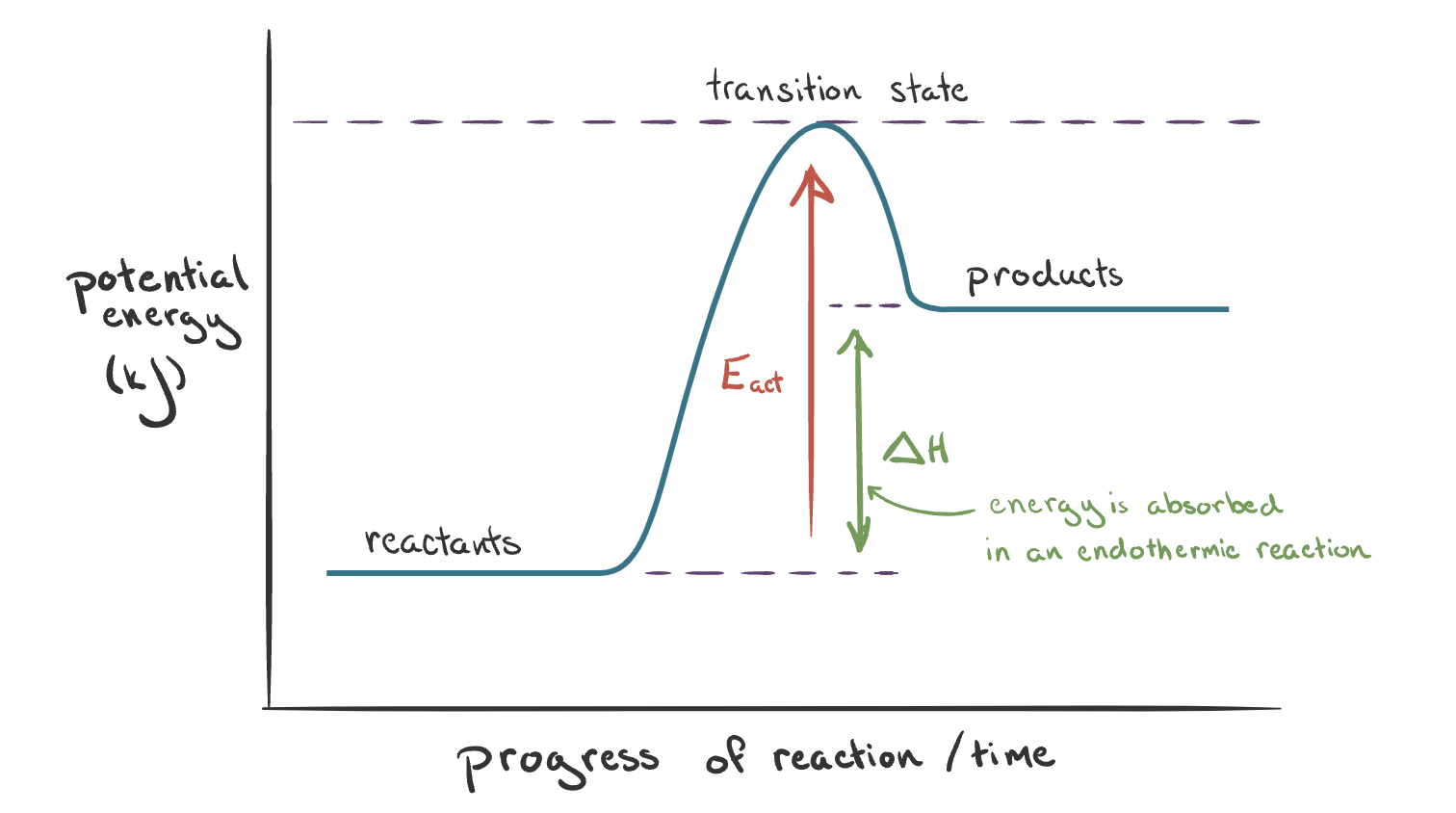
The reaction pathway diagram for an endothermic reaction. Explain.
Energy is absorbed by substances (reactants) from surroundings:
The enthalpy of products > reactants
∆H is positive (arrow points upwards)
Define standard conditions.
Explain why it is required.
State the standard conditions.
A pressure of 101kPa and a temperature of 298K (25 degrees celsius)
In order to make a fair comparison of enthalpy changes, the same conditions must be used.
They are:
A pressure of 101kPa (approx. atmospheric pressure)
A temperature of 298K (25 degrees celsius) - room temp.
Each substance involved is in its normal physical state at 101kPa and 298K.
And any solution used must have a conc. of 1moldm-3 .
What are the 7 diff. types of enthalpy changes?
∆H of reaction
∆H of formation
∆H of combustion
∆H of neutralisation
∆H of hydration
∆H of solution
∆H of atomisation
Define Standard ∆H of Reaction (∆Hr°).
State its properties.
Give an example.
The ∆H when the amount of reactants as given by the stoichometric equation react to form products under standard conditions.
Properties:
Can be exo/endothermic
All reactants and products are in their standard states.
H2(g) + ½ O2(g) → H2O(l) ∆Hr° = -286kJmol-1
2H2(g) + O2(g) → 2H2O(l) ∆Hr° = -572kJmol-1 (exactly double)
Define Standard ∆H of Formation (∆Hf°).
State its properties.
Give an example.
The ∆H when 1 mole of a compound is formed from its elements under standard conditions.
Properties
Can be exo/endothermic
All reactants and products are in their standard states.
The ∆Hf° of an element is always zero (if at standard state).
2Fe(s) + 1 ½ O2(g) → Fe2O3(s) ∆Hf° = -825.2 kJmol-1
C(graphite) + 2S(s) → CS2(l) ∆Hf° = +98.7kJmol-1
Define Standard ∆H of combustion (∆Hc°).
State its properties.
The ∆H when 1 mole of a compound is is completely burned with excess oxygen under standard conditions.
Properties
exothermic
All reactants and products are in their standard states.
substance being combusted can be element/compound.
Define Standard ∆H of Neutralisation (∆Hn°).
State its properties.
The ∆H when 1 mole of water is formed by the reaction of an acid and alkali under standard conditions.
Properties
exothermic
Ionic equation:
H+(aq) + OH-(aq) → H2O(l)
Other ions are spectator ions (doesn’t take part in reaction)
Define Standard ∆H of Hydration (∆Hhyd°).
State its properties.
The ∆H when 1 mole of a hydrated salt is formed from 1 mole of an anhydrous salt under standard conditions.
Properties
All reactants and products are in their standard states.
Define Standard ∆H of Solution (∆Hsol°).
State its properties.
The ∆H when 1 mole of a solute (ionic) dissolves in a solvent (water) to make an infinitely dilute solution under standard conditions.
Properties
Can be exo/endothermic
All reactants and products are in their standard states.
An infinitely dilute solution is when a solute in dissolved in a large amount of water, in such a way that under further dilution there is no ∆H.
Define Standard ∆H of Atomisation (∆Hat°).
State its properties.
The ∆H when 1 mole of gaseous atoms are formed from its elements under standard conditions.
Properties
endothermic
All reactants and products are in their standard states.
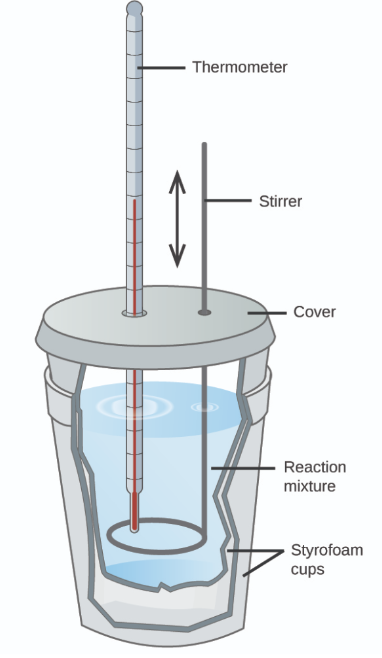
What technique can be used to measure enthalpy change.
Describe the procedure
State how enthalpy change is found using formulas.
Calorimetry (uses a calorimeter)
Procedure:
Add known amounts of reactants and known volume of liquids.
Record temperature change of liquid as reaction occurs (accuracy of thermometer must be 0.1/0.2).
Calorimetry relies on the fact that it takes 4.18J of energy to raise the temp. of 1g of water by 1°C - also known as specific heat capacity.
First calculate the heat transferred for the known no. of moles of reactants in calorimeter:
Specific Heat Capacity formula
q(J) = m(g) × C(J/g°C) × ∆T(°C)
Next calculate the enthalpy change per mole
∆H = -q/n
temp. rise (q gets +ve no.) → exothermic ∆H is -ve.
temp. fall (q gets -ve no.) → endothermic ∆H is +ve.
What assumptions are made when calculating ∆H using a calorimeter?
The density of water is 1gcm-3, so 1cm3 of water = 1g.
aqueous solutions of alkali, acids and salts are mostly water:
consider 1cm3 of solution 1g.
consider that solutions have same S.H.C as water.
State the law of conservation of energy.
State how this relates to chemical reactions.
Energy cannot be created or destroyed, only transferred from one form to another.
The total energy of the chemicals and the surroundings are always constant.
Define Hess’s Law
The ∆H of a chemical reaction is independent of the route it takes as long as the initial and final conditions are the same. And the reactants and products are in the same physical state for each route.
The ∆H for the direct route is the same as the ∆H of the indirect route.
When is Hess’s law used?
When ∆H cannot be found using experiments like calorimetry.
The difference between bond breaking and making.
Bond breaking requires energy - endothermic
Energy needed to break the attractive forces holding atoms in a molecule together
Bond making releases energy - exothermic
In terms of bond breaking and making, explain how to determine if a reaction is endo/exothermic.
If energy required to break bonds > energy released when bonds are made:
ENDOTHERMIC
The reaction absorbs energy
If energy required to break bonds < energy released when bonds are made:
EXOTHERMIC
The reaction releases energy
Are all bonds broken in a reaction. Expand.
No. Only some bonds of reactants are broken and new ones are formed in a specific sequence.
Define exact bond energy.
The amount of energy required to break 1 mole of a specific covalent bond of a named molecule in its gaseous state.
This value is +ve, as the bond BREAKS.
Define and explain average bond energies. Give an example.
The average amount of energy required to break 1 mole of a specific bond from a variety of different molecules in the gaseous state.
Bond energy is affected by other atoms in the molecule, as the specific bond is present in diff. environments.
Even identical bonds in the same molecule can have diff. values.
Example - more energy is required to break 1st O-H bond in water than the second.