Prac 9 - Energy produced by fuels
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
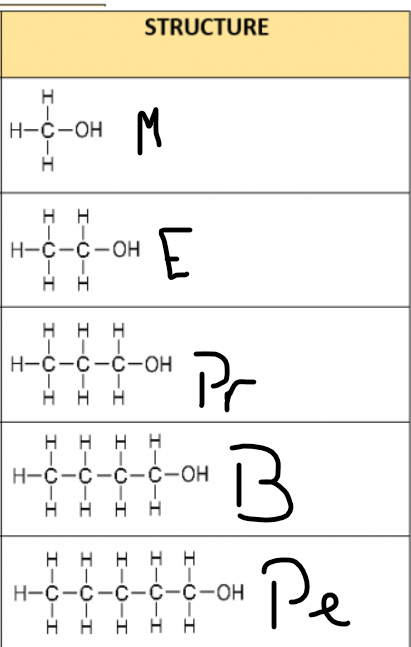
Name the first five alchohols and their formula/structure?
1) Methane: CH3OH
2) Ethanol: C2H5OH
3) Propan-1-ol: C3H7OH
4) Butan-1-ol: C4H9OH
5) Pentan-1-ol: C5H11OH
How does the number of carbon atoms affect the energy released?
More carbon atoms:
Longer chains
More C-C and C-H bonds to break
More bonds will be made in CO2 + H2O
More energy is released, making the extra bonds in CO2 + H2O than is needed to break the extra C-C and C-H bonds; therefore, this energy is released as heat.
So, the higher the number of carbons, the higher the amount of energy released per gram/mole of alchol.alcohol
What is enthalpy change?
It is the change in heat energy.
It is the measure of energy released per mole of alcohol.
Looking at the mass used to heat up the water is not sufficient because they are different molecules.
[-
We need to work out mass used per mole of alcohol.
How do you work out enthalpy change per mole?
1) Write the formula of the molecule: e.g. CO2 or H2O.
2) Use the periodic table to work out the relative molecular mass (Mr):
C = 12, H = 1, O =1 6
CO2 Mr = (1 × 12) + (2 × 16) = 44
3) Calculate heat energy released: E = mc0
4) Calculate heat energy per mole:
E = heat energy x relative molecular mass/mass of fuel burnt.
What is the relative molecular mass of Methanol?
Methanol (CH3OH)
Mr = 32
(12 × 1) + (1 × 4) + (1 × 16)
C = 12, H = 1, O =16.
What is the relative molecular mass of Ethanol?
Ethanol (C2H5OH)
Mr = 46
(12 × 2) + (1 × 6) + (1 × 16)
C = 12, H = 1, O =16.
What is the relative molecular mass of Propan-1-ol?
Propan-1-ol (C3H7OH)
Mr = 60
(12 × 3) + (1 × 8) + (1 × 16)
C = 12, H = 1, O =16.
What is the relative molecular mass of Butan-1-ol?
Butan-1-ol (C4H9OH)
Mr = 74
(12 × 4) + (1 × 10) + (1 × 16)
C = 12, H = 1, O =16.
What is the relative molecular mass of Pentan-1-ol?
Pentan-1-ol (C5H11OH)
Mr = 88
(12 × 5) + (1 × 12) + (1 × 16)
C = 12, H = 1, O =16.
What is the aim of this practical?
To investigate the energy released by different alcohols (with different length Hydrocarbon chains) when heating up the same volume of water by the same temperature.
What is the hypothesis?
As the length of hydrocarbons increases, the less mass of alcohol will be needed to burn to raise the temperature of the water by 30oc.
This happens because…
As the alcohol increases in length, there are more C-C and C-H bonds in the reactants and more bonds in the products of CO2 and H2O are formed.
Breaking the bonds in the reactants requires less energy than is released when the bonds in the products are formed and so the energy is released as heat.
What are the independent variables?
The different alcohols: Methanol, Ethanol, Propan-1-ol, Butan-1-ol, Pentan-1-ol.
What are the dependent variables?
Quantitative: Mass (g) of alcohol used
Qualitative observations: the colour and size of flame/soothiness/carbon residue.
What are the control variables?
Weighing with lid on
Same temperature rise (30oc)
Height of calorimeter above the spirit burner
Same volume of water (50ml)
Same digital thermometer.
What are the Hazards, Risks, Precautions, Emergency Procedure (H&S)
Hazard - Risk - Precautions - EP
Methanol - Toxic if ingested/causes blindness - Wear goggles/lab coats/do not touch wick - First aid kit if burnt
Alcohols - Flammable - Alcohols are supplied & used at all times in glass burners with secure and intact wicks/Alcohol burners are extinguished using the cap at all times. -
Glassware - Smash/injuries - Keep away from the bench edge - Contact first aid/teacher for clean up.
Calorimeter - Burns - When the calorimeter has been heated, do not touch until cool or use heat-proof gloves to empty - Run area under cold water.
Hot water - Burns if spilt/causes injuries - contact first aid/teacher to clean up.
What equipment ia used ?
Digital thermometer - to measure the temperature of water
Clamp and stand to hold the calorimeter
Calorimeter & lid - to hold the water and keep O2 in
Measuring cylinder (50ml) - to measure 50ml of water
Mass balance - to weigh the mass of each alcohol spirit burner with the lid on before and after being lit.
Matches - to light the fuel
Stop clock - to record when the water increased by 30 degrees
Heat-proof mat - to ensure no damage to the benches
What is the method?
1) Set up the equipment as per the diagram
2) Add 50ml of water to the calorimeter can using a 50ml measuring cylinder
3) Place the lid on the calorimeter and place the thermometer in the water
4) Record the temperature of water in the table
5) Weigh the mass of the ethanol spirit burner (with the lid on) using the mass balance and record the mass (g) of the fuel to 2dp.
6) Place the spirit burner under the calorimeter and light the fuel.
7) When the water temperature has risen by 30 degrees, place the lid back on the fuel to extinguish the flame.
8) Weigh the mass of the spirit burner and fuel, recording the final mass (g)
9) Clean the calorimeter of any soot
10) Repeat the experiment to get 3 repeats
11) Repeat the steps above with each of the 4 fuels
What was the graph like?
Bar chart
x-axis titled alchols
y-axis Heat energy per mole (kJ/mol)
What was the conclusion?
Same hypothesis
Just add Butan-1-ol and Pentan-1-ol would have burnt with a bigger orange flame and would have created more soot as they have more incomplete combustion.