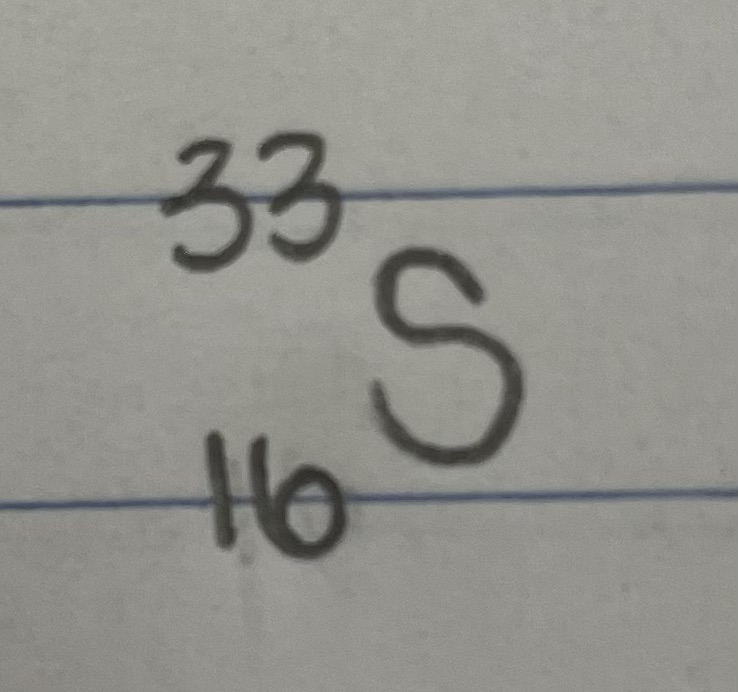Nuclear Physics 10: pg. 291 Practice Problems 3-4
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
An element is analyzed and found to have a mass number of 37. An atom contains 20 neutrons in its nucleus. State the number of protons.
17 protons
An element is analyzed and found to have a mass number of 37. An atom contains 20 neutrons in its nucleus. State the name of the isotope.
chlorine-37
An element is analyzed and found to have a mass number of 37. An atom contains 20 neutrons in its nucleus. State the standard atomic notation.
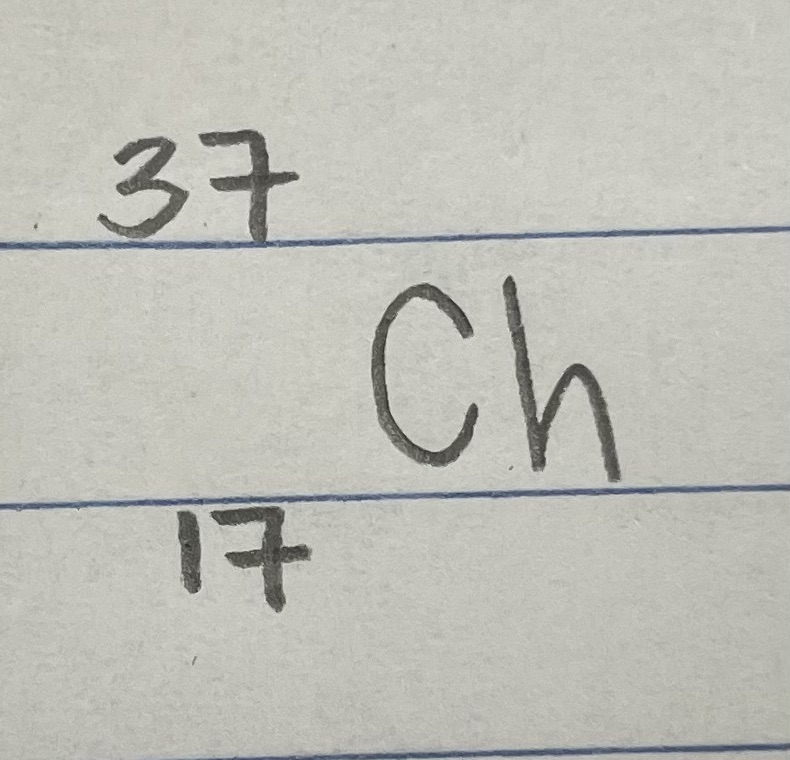
For an isotope containing 16 protons and 17 neutrons, state the atomic number.
Atomic Number = 16
For an isotope containing 16 protons and 17 neutrons, state the mass number.
Mass Number = 33
For an isotope containing 16 protons and 17 neutrons, state the name of the isotope.
The isotope is called sulfur-33.
For an isotope containing 16 protons and 17 neutrons, state the standard atomic notation.