Toxins
1/154
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
155 Terms
Chloroform
Bioactivated by P450 into unstable trichloromethanol, forms phosgene (COCl2 ) with HCl which reacts with proteins and results in necrosis
NSAIDs
Used for pain management (analgesia) and anti-inflammation • Ibuprofen, aspirin, Voltaren • Most NSAIDs inhibit cyclooxygenase-1 (COX-1) and -2 (COX-2) -> inhibited prostaglandin synthesis ->inhibited afferent vasodilation-> constriction of the afferent arteriole -> decreased GFR • Can cause kidney toxicity: - acute tubular necrosis - acute interstitial nephritis (immune mediated?) - papillary necrosis
Average cell membrane thickness
Thin, 7-9 nm
What does transport rely on?
size, charge, solubility, lipophilicity, concentration gradient
Size of hydrophilic molecules which can pass through aquous pores
≤ 600 Da, e.g.ethanol
Are the majority of toxicants lipid or water soluble?
Lipid soluble, large organic molecules
Lipid solubility is expressed with the octanol/water coefficient KOW or LogP and transport rate is proportional to it.
Examples of super lipid soluble toxins
TCDD, DDT
How do ionised acids and bases pass through membranes?
via aqueous pores or active transport
Fick’s law
J = kD . A . (C1-C2)/x
• J is the rate of diffusion • k is the partition coefficient (lipid solubility, P) • D is the diffusion coefficient (temp, viscosity of soltn…) • A is the area of absorption • X is membrane thickness • C1-C2 is the concentration gradient x
High kp (kD/x)
few cell layers, lipophilic compounds
small (< 180 g/mole), nonionized lipid soluble compounds
Low kp
many cell layers, ionized compounds
water soluble compounds > 180 g/mole
Filtration
Driven by hydrostatic pressure through membrane pores, small molecules dissolved in water
Glomeruli in the kidney, 70 nm, molecules < 60000 Da
Regular cells < 4 nm, molecules < 300-400 Da
Active transporters in GI tract
Aid in uptake of nucleotides, metals, di- and tri-peptides
Facilitated diffusion
sugar and amino acid transport
GI tract physiological factors
• Surface area – very big > 300 m2 • Blood flow rate – very high
Is lipid solubility or particle size more important in the GI tract?
Particle size
Factors that decrease bioavailability
• Enzymatic degradation inside the digestive tract –proteases and peptidases split proteins into small peptides and amino acids. –lipases split fat into three fatty acids and a glycerol molecule. –amylases split carbohydrates such as starch and sugars into simple sugars such as glucose. –nucleases split nucleic acids into nucleotides.
Big particle size
Short residence time
Biotransformation in epithelial cells of the intestine
Biotransformation in the liver
Elimination in the lungs
First pass effect (presystemic elimination)
e biotransformation in the epithelial cells of GI tract or in the liver is called
Newborn GI tract
Higher pH
Different bacterial flora
Species differences GI tract
intestine length
pH
bacterial species - number and location (monkey higher than man)
Area of skin
2m2 with 1% pores
7 layers to reach blood vessels
Rate-limiting is stratum corneum, keratinized cells
Stratus Corneum
replenishes every 3-4 weeks
Dehydrated, Polymerized,
Cell membrane double in thickness
Aqueous, fluid medium → dry, semisolid medium
passive diffusion
Permeability is a combination of both diffusivity and thickness of stratum corneum
Factors that affect stratum corneum absorption
Calluses – decreases permeability
Hydration – can increase permeability up to 30-fold
Peeling or “skin care” – increases permeability
Burns – severely increases the permeability
Differential stratum corneum toxicity
Big species differences
Rats and rabbits more permeable
Cats less permeable
Guinea pigs, pigs and monkeys are similar to human
Humans usually have thicker stratum corneum, but less protective hair.
Pores from sweat and sebaceous glands vary
Membrane size in the lungs
No. of alveoli: 700 x 106 Area of alveolar membrane: 60 – 80 m2
What causes toxicity in the nose?
Formaldehyde - Water soluble gases and highly reactive gases are retained in the mucus of the respiratory system e.g. nose acts as scrubber
Factors affecting lung absorbance
Water solubility - low absorbed better
(ionised molecules have lower volatility to less in the air)
High blood flow
Type 1 pneumocytes very thin
blood-to-gas-partition coefficient (Pf )
Chloroform = 15, Ethylene = 0.14
Low pf limited by perfusion
High pf limited by ventilation
Particle size lung absorption
Particles > 5 µm, nasopharyngeal region, can dissolve in mucus and be absorbed. Most are cleared by sneezing, wiping
• 2-5 µm, tracheobronchial regions of lung, cleared by movements from cilia. Swallowed and absorbed in the GI tract.
• Particles < 1 µm, alveolar sacs, may be absorbed into the blood or cleared by mucociliary escalator, phagocytosis by macrophages, or penetrate into the lymphatic system.
Distribution
Very polar and moderate to big ions (50 kDa or more) needs help by active transport mechanisms
Storage proteins
Plasma proteins can bind endo- and exogenous compounds
Metals • Transferrin – beta globulin, binds iron (Fe2+) • Ceruloplasmin – alpha globulin, binds copper (Cu2+)
Lipid-soluble compounds • Alfa- and beta- lipoproteins binds vitamins, cholesterol, steroid hormones
What does albumin bind?
Endogenous binding: long-chain fatty acids, bilirubin, adenosine, thyroxine (Evans blue binds almost 100% to albumin)
Storage in the liver and kidney
Ligandin (binding protein for steroids and bilirubin) has high affinity for organic acids • Metallothionein binds heavy metals (Cd, Hg, Zn)
Storage in fat
Organic environmental pollutants are very lipophilic
DDT • Chlordane • PCBs (polychlorinated biphenyls) • PBDEs (polybrominated diphenyl ethers)
Rapid transport over cell membranes and uptake in tissues, Highly concentrated in body fat
Storage in bones
Fluoride (F- ) displaces OH-
Strontium2+ and lead2+ displace Ca2+
What is actively transported in the placenta
Ions, calcium and iron
Amino acids
Vitamins
Vital sugars
Protective mechanisms of the placenta
Although toxicants in mother and fetus are usually in the same concentrations,
The fetus is protected from toxicants by active transport systems – OCT3 - organic cation transporter – OAT4 – organic anion transporter – OATP2 – organic-anion transporting poylypetide – MDR1 – multidrug-resistant protein – MRP1, 2, 3 – multiresistant drug prot – BCRP - Breast Cancer Resistance Protein The placenta also have biotransformation systems, preventing some chemicals from reaching the fetus
Fetal differences
lower plasma protein concentration in fetus
BBB not fully developed
Lower body fat content in fetus
Kidney filtration sizes
The kidney receives about 25 % of the cardiac output • 20 % of that is filtered at the glomeruli! • The capillaries of glomeruli have large pores (70 nm) – Molecules up to 60000 Da can be filtered out of the blood – Proteins smaller than albumin – Degree of plasma protein binding affects glomerular filtration, since complexes of xenobiotic-protein are to big to be filtered through the pores
pH based treatments
Phenobarbital is a weak organic acid (pKa 7.2) – NaHCO3 à increases pH à increases ionized form à increased excretion
Salicylate (an important active metabolite of aspirin (acetylsalicylic acid) excretion can also be increased with sodium bicarbonate
Active renal secretion
Can eliminate protein bound toxicants, but can be affected by competitive inhibition
Reuptake in proximal tubule
Cadmium binds to metallothionein
complex reabsorbed
→ toxic effects in tubular cells
Differential excretion
Organic acid transport system that secretes penicillin is underdeveloped in premature neonates (20% excretion)
Excretion of weak organic acids vary a lot due to differences in urine pH
[Filtration in glomeruli can differ due to protein-binding and differences in plasma proteins between species
– Types of active transporters and affinity of the transporters varies
– Species differences in biotransformation]
What type of materials go through fecal excretion
– Indigestible material
– Nutrients
– Drugs and other xenobiotics
– Exceptions are macromolecules and fully ionized high molecular weight compounds (polymers)
– Rare that 100% is absorbed
Basically, anything that is not absorbed and bile output of liver
Enterohepatic circulation
Glucuronidated conjugates are excreted in the bile – Intestinal microflora contains β-glucuronidase that hydrolyses the complex
Sulfate conjugates also undergo enterohepatic circulation – Intestinal microflora contains aryl sulfatases that hydrolyses conjugates
Arsenic
Bile to plasma ratio
Class A: Ratio nearly 1. Ex. Na, K, glucose, Hg, Th, Cs, Co.
Class B: Ratio greater than 1 (10-1000). Generally compounds rapidly excreted in bile. Ex. bile acids, bilirubin, lead, arsenic, manganese.
Class C: Ratio below 1. Ex. inulin, albumin, Zn, Fe, Au, Cr
Class B excretion
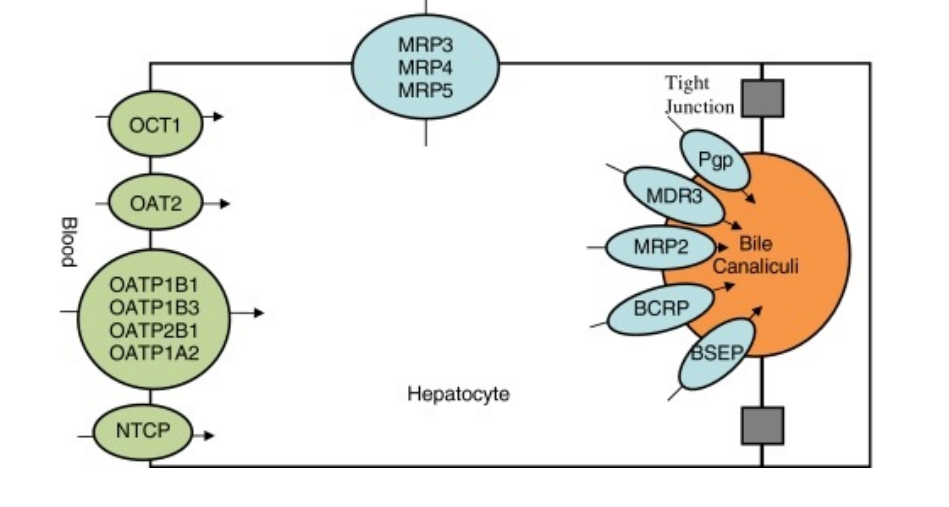
Class B compounds are actively transported from plasma to hepatocytes and from hepatocytes to bile – The same types of transporters are present in hepatocytes (OATP, OAT, OCT, MRP, MDR) – Ntcp (sodium dependent taurochlorate peptide) is specific for hepatocytes and transports bile salts into the liver – Lst (liver specific transporter) transport compounds into the liver – Bsep (bile salt excretory protein) is also specific for hepatocytes and transports bile salts out of the liver and into bile canaliculi
What type of compounds are excreted in bile
Usually low-molecular-weight compounds are poorly excreted in bile
– Compounds or conjugates with molecular weights over 325 are excreted in bile
– Large species differences but excretion is more compound-specific than species-specific
– Compounds excreted in bile enter the intestine and can either be excreted with feces or be reabsorbed
Increasing biliary excretion
Phenobarbital can increase the flow of bile – Increases the hepatic excretory function- good for methymercury excretion
Some steroids increase bile production à enhance excretion – Cardiac glycosides are more effectively eliminated
Differential biliary excretion
– Age: Newborns do not have a fully developed hepatic excretory system. » Hepatic excretory function can be promoted by microsomal enzyme inducers
– Species: Different species excrete different compounds differently. Both route and time can differ.
Intestinal excretion
Passive diffusion, slow, increased by increasing lipid concentration in small intestine
Microflora can biotransform usually favouring reabsorption
Milk excretion
Milk has pH 6.5, facilitating excretion of basic compounds (ionised at low pH) • Acidic compounds have lower concentration in milk than in blood • Milk is contains 3-4 % fat (lipids)
• Lipid-soluble compounds diffuse with lipids into mammary cells
• Many environmental pollutants are lipid-soluble and are excreted in milk, DDT, PCBs, PBDEs, dioxins
• Metals similar to calcium (lead) and chelating agents can also be excreted in milk
Seals- 50% fat in milk
Humans- 4% fat in milk
Cytochrome P450
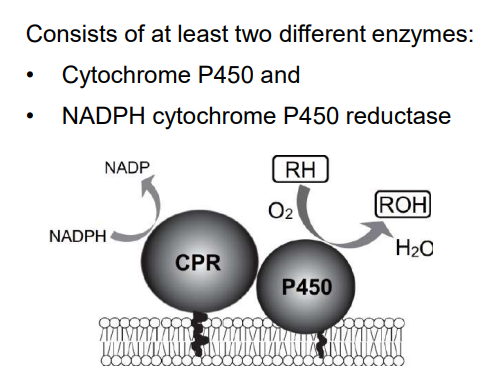
Phase I examples
Oseltamivir→ Oseltamivir carboxylate (Hydrolysis)
Organophosphates (Hydrolysis)
Acetylsalicylic acid → salicyclic acid (hydrolysis of prodrug)
Quinones to non-toxic hyroquinones (NQO1 and NQO2 reduction)
Quinones to toxic semiquinone free radical (Cytochrome P450 reductase, in low oxygen)
Serotonin → amides, primary and secondary alcohols (MAO, oxidation)
[MAO located in • Brain; • Outer mitochondrial membrane in liver, kidney, intestines, blood platelets]
Reactions of Cytochrome P450
Hydroxylation of Aliphatic carbons
Dehydrogenation
Cleavage of esters
Activation of P450
Gene duplications
Exposure to drugs and other xenobiotics that directly induce new synthesis of P450
Activation of an already existing enzyme
Inhibition of P450
Mutations
Viral infections or inflammation
Exposure to xenobiotics
P450 induction example
TCDD - AhR binding (ARNT coenzyme)
Phase II reactions
• Glucuronidation
• Sulfonation
• Methylation
• Acetylation
• Glutathione Conjugation.
Phase II reaction example
Methylation of noradrenaline and histamine
Weight of the liver
1200-1500g (largest gland in body)
Functions of liver
Metabolic homeostasis : Control of synthesis and utilization of carbohydrates, lipids and proteins as well as metabolism of xenobiotics
Vascular functions such as formation of lymph and the hepatic phagocytic functions
Secretory and excretory functions, particularly with respect to the synthesis and secretion of bile
Hepatic blood supply
About 75-80% of hepatic blood flow is from the portal vein and about 20-25% from the hepatic artery.
Sinusoid physiology
Fenestrae measure between 100 and 200 nm in diameter Allow molecules < 250 KDa
Get smaller and stiffer with age
Kupffer cells
specialized macrophages lining the walls of the sinusoids
•Stellate cells (Ito cells; fat-storing cells)
Found in the perisinusoidal space (space of Disse; a small area between the sinusoids and hepatocytes). They store vitamin A and fat and produce extracellular matrix and collagen. The stellate cell (Ito cell) is the major cell type involved in liver fibrosis, which is the formation of scar tissue in response to liver damage.
What percentage of liver volume is hepatocytes?
78%
Zone 1
periportal: Hepatocytes are exposed to the highest concentration of oxygen, hormones, nutrients, and xenobiotics coming from the intestine (portal vein) or the systemic circulation (hepatic arteries), Fe overload can occur
Zone 3
centrilobular: Located around central vein where blood exits (low concentration of oxygen)
Determining factor of liver cell injury
Balance of Phase I and Phase II reactions
Zone 3 damage
•Many hepatotoxicants elicit centrilobular (zone 3) damage because CYPs (especially CYP2E1) are preferentially localized in centrilobular hepatocytes (example of a chemical causing centrilobular damage: paracetamol/acetaminophen)
•High levels of CYPs coupled with a reverse distribution for glutathione makes centrilobular hepatocytes especially susceptible to reactive electrophiles
Paracetamol/acetaminophen toxicity
•A widely used analgesic (pain reliever) and antipyretic (fever reducer) •Generally safe for use at recommended doses (1,000 mg per single dose and up to 4,000 mg per day for adults)
•Acute overdoses of paracetamol can cause potentially fatal liver damage (centrilobular necrosis)
•Chronic alcohol abuse increases the risk (Ethanol induces CYP2E1)
Ethanol metabolism
Oxidised
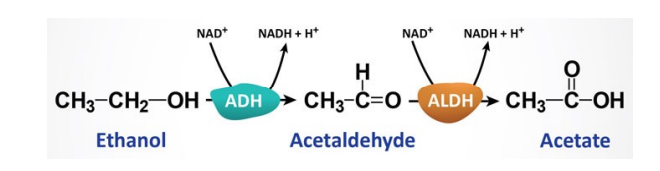
ADH
Alcohol dehydrogenase (ADH)
- Genetic polymorphisms very relevant here.
- Bulk of metabolism initiated with this enzyme.
- Requires NAD+
- Produces Acetaldehyde
- ALDH then oxidizes acetaldehyde in the mitochondria.
Microsomal ethanol oxidizing system (MEOS)
- Involves primarily CYP2E1 (and CYP1A2/CYP3A4)
- Induced through chronic exposure
- Explains increased tolerance through exposure
- Impacts metabolism of other chemicals (paracetamol to NAPQI/warfarin/diazepam
Ethanol toxicity
NAD+ oxidises other species, but it acts as a protective/repairing agent for DNA. Excess NADH/NAD+ ratio reduces the amount of free NAD+ available to aid ALDH in further metabolising acetaldehyde to acetate. This means there is a greater load of acetaldehyde in the system, binding to and damaging mitochondrial DNA by forming adducts. This can lead to impaired glucose production, mitophagy, apoptosis etc. ROS are also produced by the microsomal ethanol oxidising system which damages membranes and DNA. MEOS regulated by CYP2E1 is upregulated by chronic ethanol exposure and therefore chronic alcohol abuse can also increases the risks associated with taking acetaminophen. • Increased lactate, triglycerides (chronic fatty liver)
Main causes of DNA damage
acetaldehydes (from ethanol), ROS, aldehydes
Carbon tetrachloride toxicity
Lipid peroxidation results in reactive aldehydes causing cell membrane and DNA damage
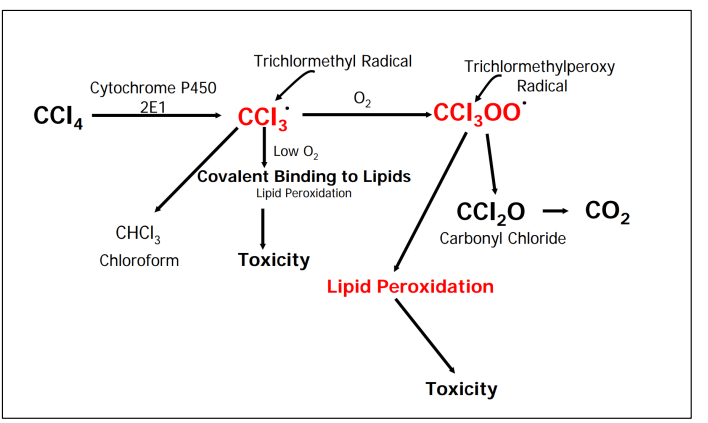
Causes of apoptosis/necrosis in liver cells
• Peroxidation
• mitochondrial damage
• Cytoskeletal damage
• Calcium influx
How is cholestasis measured?
serum concentrations of conjugated bilirubin and bile salts are the most commonly measured to determine if present.
Bilirubin excretion
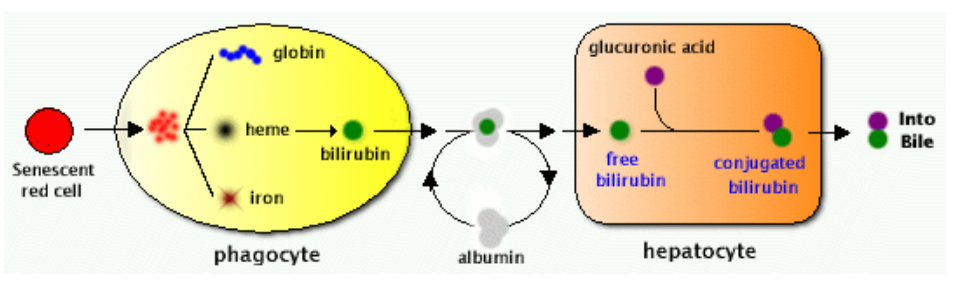
Fibrosis
Characterized by the accumulation of large numbers of collagen fibers •Stellate cells (Ito cells) located in the space of Disse play a critical role in the progression of fibrosis
Cirrhosis
Advanced fibrosis results in scarring of the liver that can lead to irreversible damage
Glomerular filtration rate
= 125 ml/min (180 L/day), sum of about 2million gomeruli
Blood flow to different sections of the kidney
Renal cortex receives 90 % of blood flow • Greater blood volume = greater risk for toxicant exposure
Renal medulla receives 6-10 % of blood flow
What percentage of salts are reabsorbed
98-99%
Kidney vulnerabilities
High blood flow
Concentration mechanisms
Active transport
High metabolic activity
Reactive metabolite production
Selective accumulation
What do juxtaglomerular cells do?
Produce renin which mediates formation of Angiotensin II – vasoconstriction
Compensatory mechanism
Renal hypertrophy
Increaed 40→60% glomerula filtration rate in remaining kidney
Mask underlying damage
Acute renal failure
Acute drop in GFR
Azotemia: increase in blood urea nitrogen and creatinine
What percentage of reabsorption happens in the proximal tubule?
60-80%
Proximal tubule toxicity
Proximal tubule
• Heavy metals:
– Hg: conjugated to glutathione or cysteine and cotransported into cells. Early signals = mitochondrial dysfunction which results in necrosis
– Cd: Targets cell-cell adhesion signaling pathways, dysfunction of tubule and consequently interstitial nephritis (inflammation) and necrosis
Halogenated compounds:
– Chloroform: bioactivated by P450 into unstable trichloromethanol, forms phosgene (COCl2 ) with HCl which reacts with proteins and results in necrosis
Mechanisms of acute renal cell injury
• Apoptosis (mitochondria driven)
• ROS – damage proteins, lipids, DNA
• Loss of ATP and therefore ion transport and ion balance
• Loss of polarity and therefore effective cell function
• Lysosomal overload and enzyme release
• Alterations to Ca2+ dynamics within the cell
• Impaired membrane function
CNS susceptibility
Lipid-rich environment
Repair mechanisms are limited (post mitotic)
High dependence on oxygen (loss = cell death)
Dependence on glucose (loss = cell death)
Receives lots of blood flow (~15%)
High metabolic rate
Many inhaled substances go straight to the brain
Neurotoxic injury may result in multiple outcomes such as: − Sensory disorders − Movement disorders − Learning disorders − Memory disorders
Diethylstilbestrol
Estrogen receptor agonist
Given to prevent miscarriage
DES-exposed daughters developed a rare form of vaginal cancer in their early 20s (1:1000 in DES exposed vs none in nonexposed)
Definition of an endocrine disruptor
An endocrine disruptor is an exogenous substance that alters the function of the endocrine system causing adverse health effects in an intact individual, its progeny or subpopulations
EDC and traditional toxicant differences
Traditional toxicant:
Immediate effect
”Dose makes the poison”
Clear effects (death, skin corrosion, eye irritation...)
EDC:
Effect visible years/decades later
Non-monotonic dose response
subtle effects (e.g. subfertility, behavioural changes)
How many hormones are there?
Approx 100 in 50 hormal signalling systems, endocrine signalling, well preserved across species
Functions of hormonal systems
In adults:
Regulation of metabolism
Regulation of water, salt and nutrient uptake
Stress response
Reproduction
During development:
Organ development,
Growth and organisation
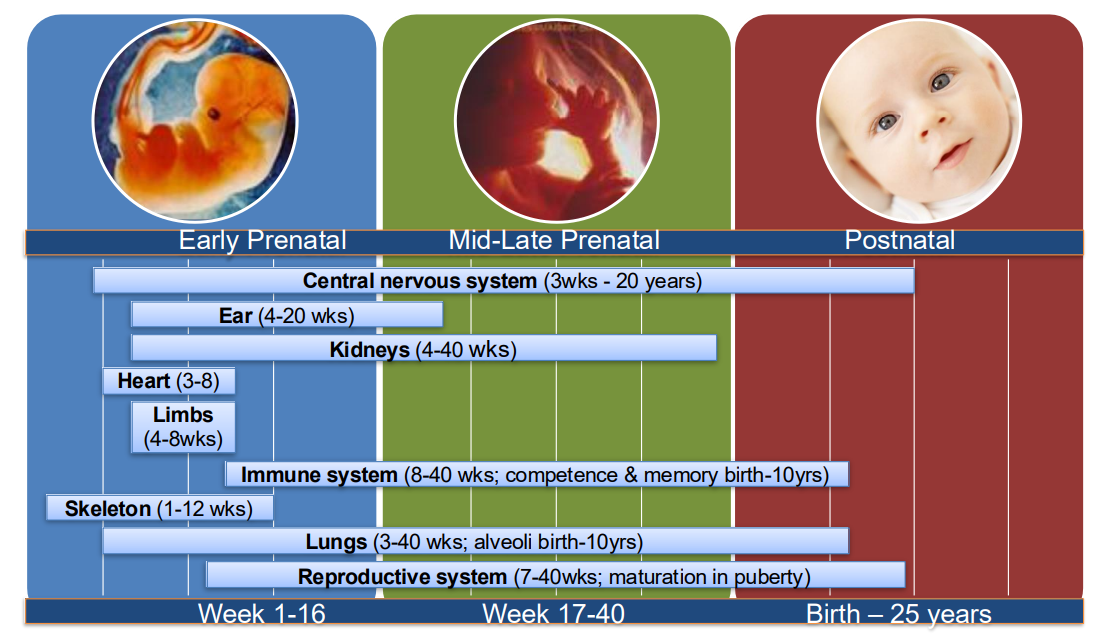
What does hormone signalling depend on?
• Hormone production and secretion
• Transport in the blood by plasma proteins
• Receptor availability and activity
• Hormone metabolism and excretion