Chemistry
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
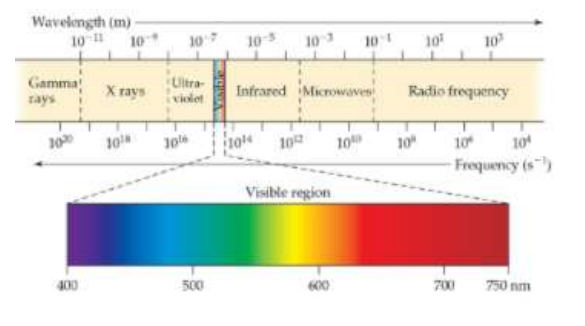
Visible Light
One type of Electromagnetic Radiation, visible to the human eye
Light
Has been Identified as both a particle and a wave
Quantized Energy and Photons
When Heated Solids emit Radiation
Max Planck
(1858-1947), A German Physicist who proposed the idea that energy can be released or absorbed by atoms. “QUANTA”
Quantum
Also known as “Fixed Amount”
Planck and Einstein
These two paved the way for understanding how electrons are arranged in atoms
1913
This is when the Danish Physicist Niels Bohr offered a theoretical explanation of line spectra, another phenomenon that had puzzled scientists during the nineteenth century.
A spectrum
Is produced when radiation from a polychromatic source is separated into its component wavelengths.
A continuous spectrum
This is the rainbow of colors, containing light of all wavelengths.
A line spectrum
This is the spectrum containing radiation of only specific wavelengths.
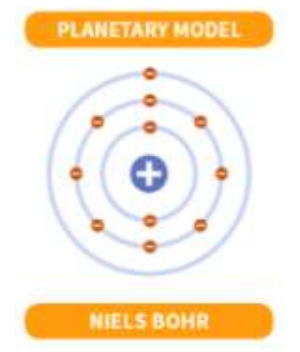
Niels Bohr
Assumed that electrons in hydrogen atoms move in circular orbits
Niels Bohr’s Planetary Model
Only orbits of certain radii, corresponding to specific energies, are permitted for the electron in a hydrogen atom.
Ground State Electrons
The Lowest Energy State
Excited State Electrons
The Highest Energy State
Louis de Broglie
(1892–1987), Suggested that an electron moving about the nucleus of an atom behaves like a wave, and therefore has a wavelength. (Wave Properties of Electron).
Werner Heisenberg
German Physicist, Proposed that the dual nature of matter places a fundamental limitation on how precisely we can know both the location and the momentum of an object at a given instant. (Uncertainty Principle).
Schrodinger’s Model
Developed a mathematical treatment into which both the wave and particle nature of matter could be incorporated: quantum mechanics.
Orbitals
The solution to Schrödinger’s equation for the hydrogen atom yields a set of wave functions.
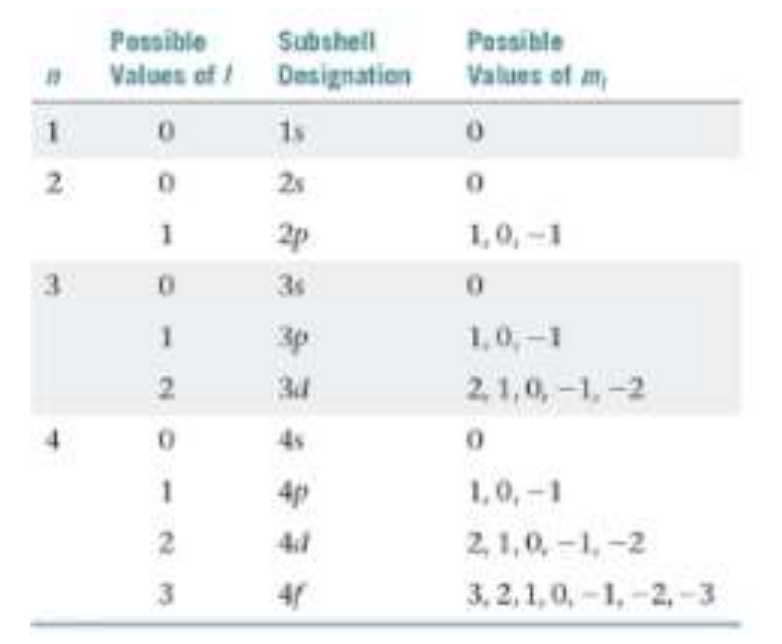
Is the set of quantum numbers n, l, ml.
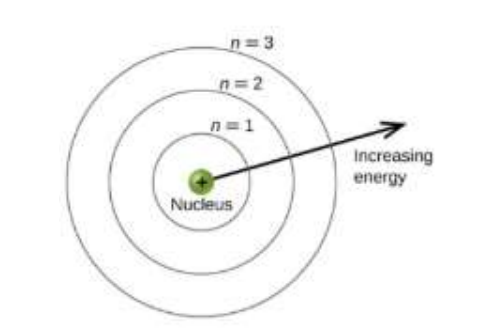
Principle Quantum Number
Also Known as “N”, Always N > 1. Describes the energy level where the electron resides in an orbital. As the orbital becomes larger, the electron spends more time further away from the nucleus.
Angular Momentum Quantum Number
Also Known as “L”, Always L cannot be equal to N. Defines the shape and is designated with the letters s,p,d,f where s=0, p=1, d=2, f=3.
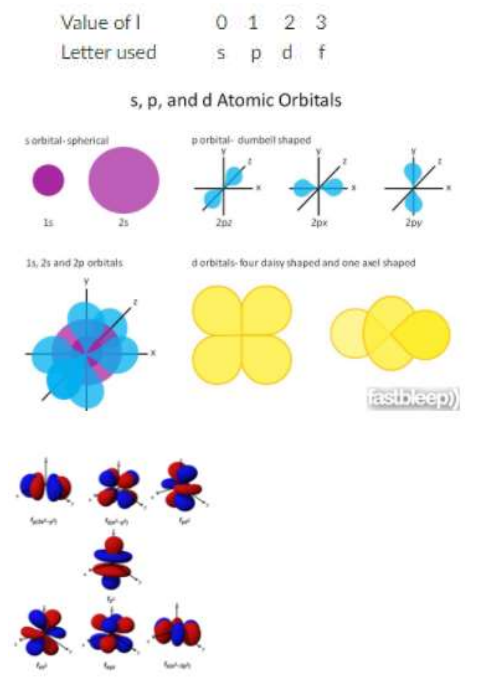
Magnetic Quantum Number
Also Known as “ML”, Always integral values between -L and L including the value zero. Describes the orientation of the orbital.
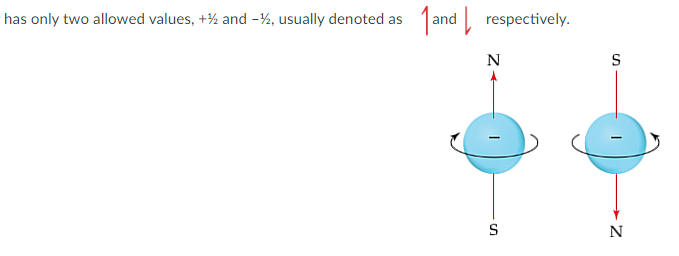
Spin Quantum Numbers
Also Known as “MS”, Always ½ (going up/positive) or -½ (going down/ negative). In 1920 It was discovered that Electrons don’t have the same spin and the spin describes the magnetic field.
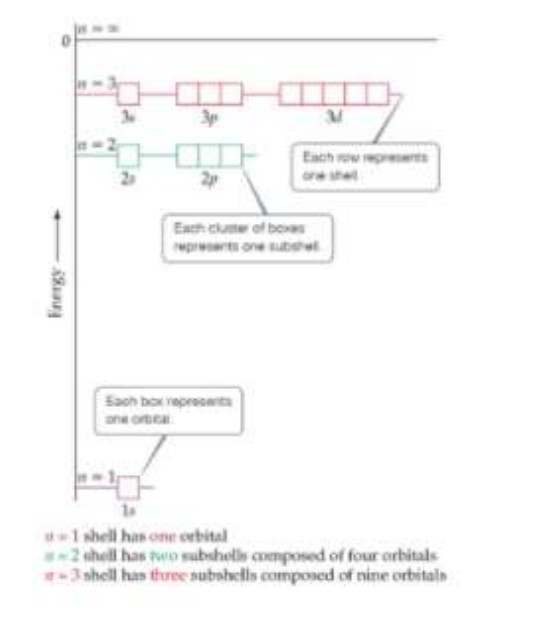
Orbitals and Their Energies
In atoms with more than one electron, not all orbitals on the same energy level are degenerate.
Paramagnetic
Has a missing set of pairs.
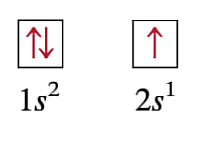
Diamagnetic
Has a full set of pairs.
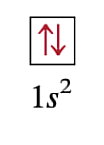
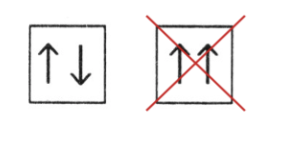
Wolfgang Pauli (Pauli’s Exclusion Principle)
(1900-1958), Austrian Born Physicist. Proposed the idea that NO two electrons can have the same set of quantum numbers (n, l, ml, ms). And that the arrows inside the boxes should always be opposite from one another.
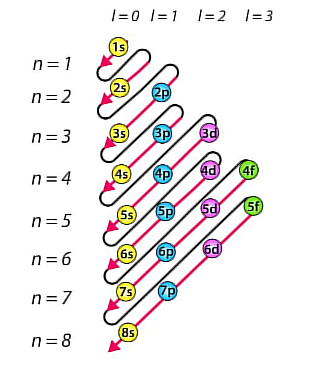
Aufbau’s Principle
Dictates the manner in which electrons are filled in the atomic orbitals of an atom in its ground state.
Hund’s Rule
Electrons occupy orbitals singly to the maximum extent possible and that these single electrons in a given subshell.
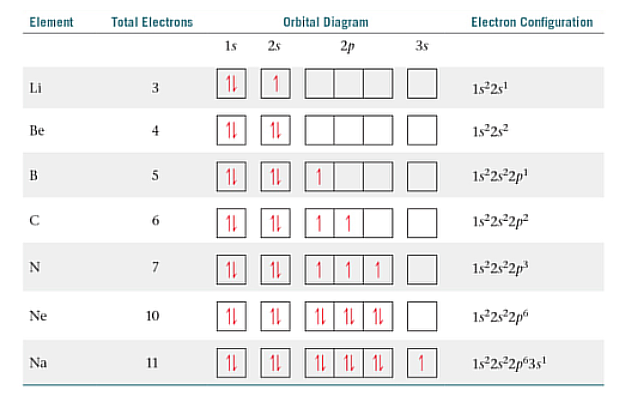
Electron Shells
This is what the collection of orbitals with the same value of n is called (Drawn as Boxes, where each box represents an orbital).
Subshell
This is what the set of orbitals that have the same n and l values is called.
Electron Configuration
The way electrons are distributed among the various orbitals of an atom.
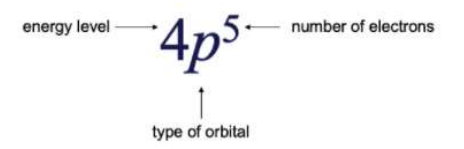
4 Indicates the Energy Level of the element (The period in which where the element lies)
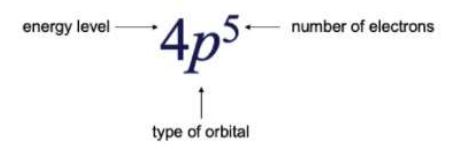
P Indicates the type of orbital of the electron lies. (S,P,D,F Blocks)
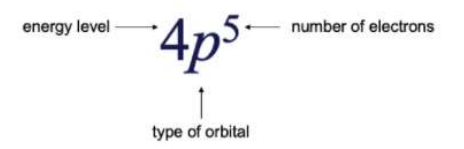
5 Represents the number of electrons present in the element.
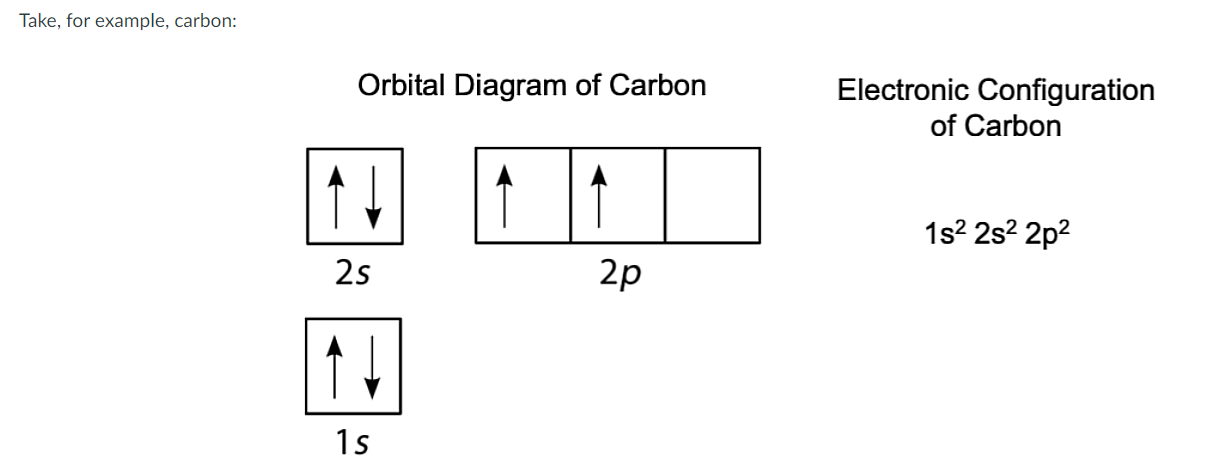
What does the Boxes Represent?
Represents the Orbital Diagram.
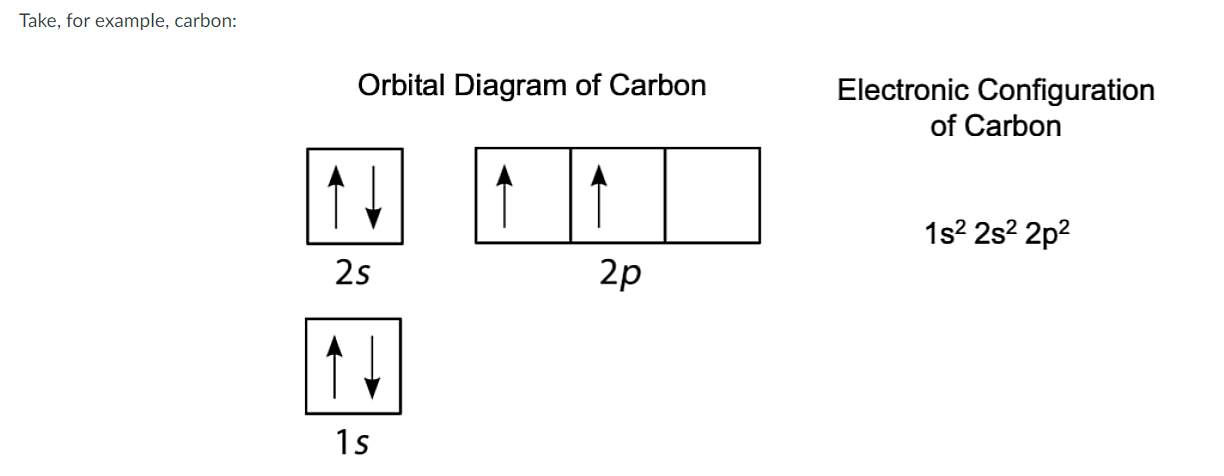
Does the representation of the spin (arrows) violate the rule of Pauli’s Exclusion Principle?
No, None of the arrows are facing in the same direction. They are all Opposite.
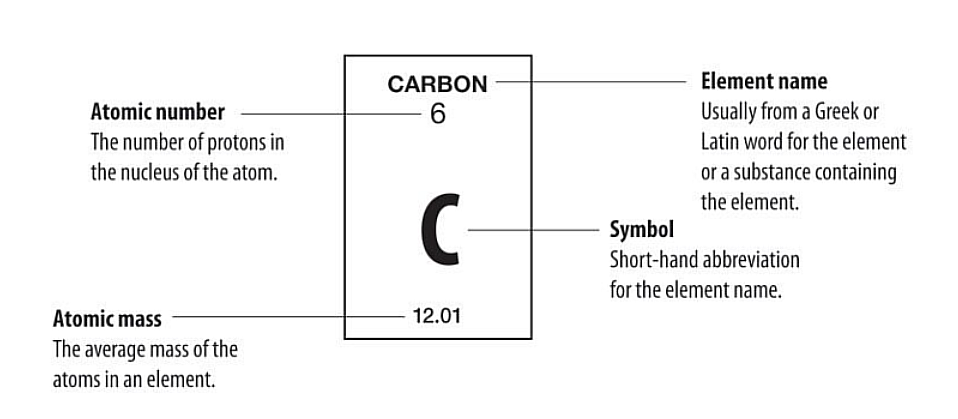
This Represents the information needed in the Periodic Table.
Periodic Table
A powerful tool for understanding and predicting the physical and chemical properties of elements.
Dmitri Mendeleev
The person most credited for the development of the Periodic Table.
Mendeleev’s Table
Was Based on atomic masses.
Ernest Rutherford
Discovered the Nuclear Atom
Henry Moseley
Developed the concept of atomic number experimentally.
Valence electrons
Are the electrons in the highest shell. The chemical reactivity of the elements is largely determined by this
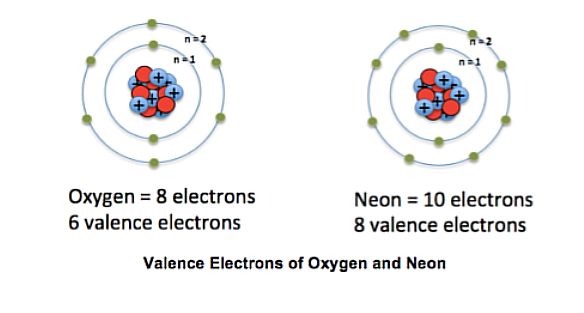
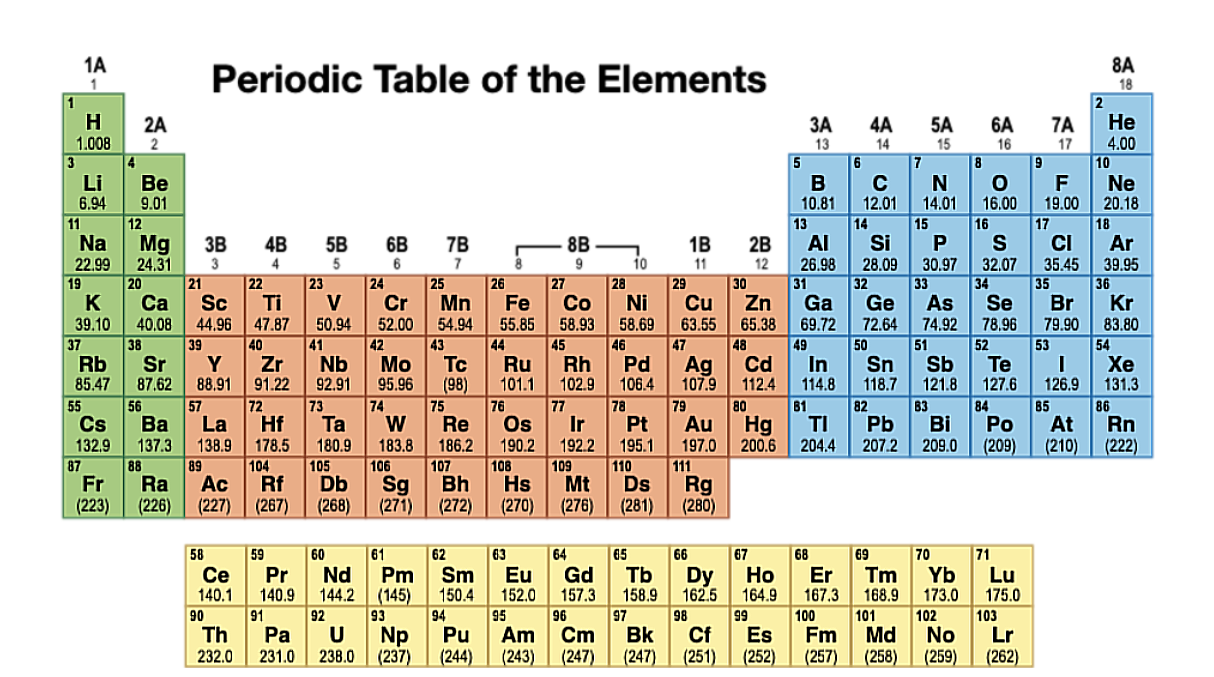
Periods
Elements are arranged in Periods (Rows), Elements in the same period have the same energy level.
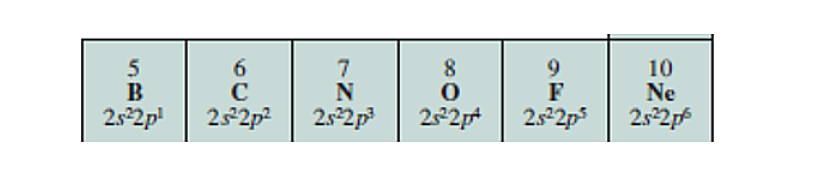
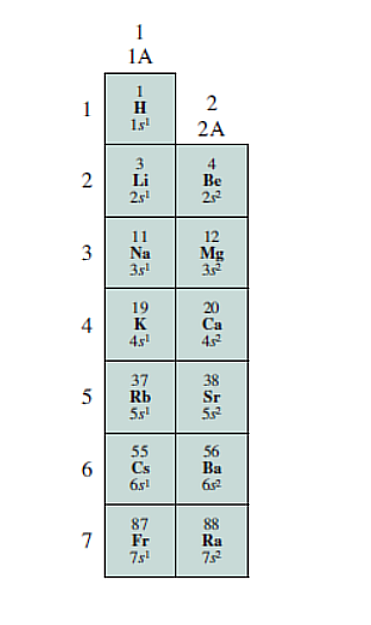
Groups
Elements are arranged in Groups (Columns), Elements in the same group have the same number and types of valence electrons.
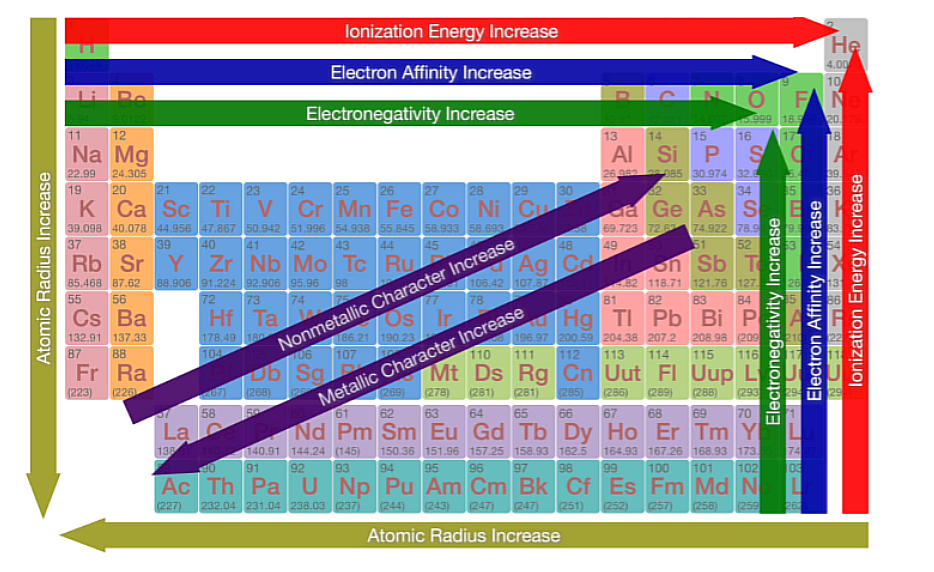
Periodic Trends
The Periodic Trends that exist within a group
Alkali Metals
Soft, metallic solids, found in the compounds in nature not in their elemental forms
Alkaline Earth Metals
Higher density and melting points than alkali metals, form +2 cations, losing the 2 valence electrons
Halogens
Typical nonmetals, highly negative electron affinities so they exist as anions in nature, react directly with metals to form metal halides
Noble gases
Helium and neon are chemically inert, completely filled ns and np subshells and result to lack of chemical reactivity, Law of Octaves: found as monatomic gases
Metals
Most of the elements in nature, shiny luster, conduct heat and electricity, malleable and ductile, solid except mercury, low ionization energies, form cations easily.
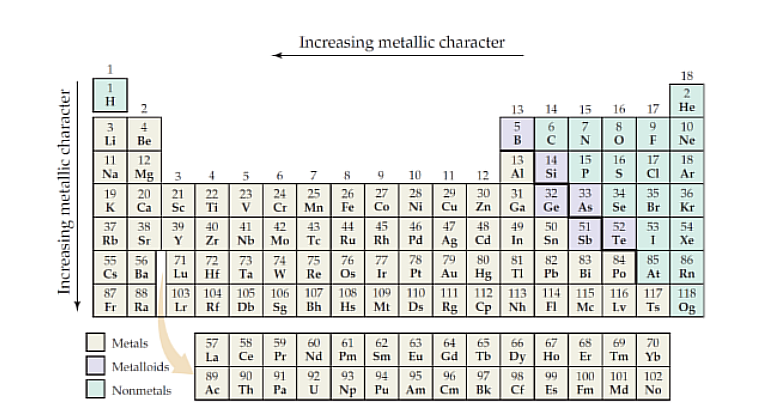
Non-Metals
Found on the right-hand side of the periodic table, solid, liquid, gas, solids are dull, brittle, poor conductors, larger negative electron affinity, form anions readily.
Metalloids
Have some characteristics of metals and some of nonmetals, some are electrical semiconductors
Periodicity
Is the repetitive pattern of property for elements based on the atomic number.
Nuclear Charge
The nuclear charge increases from left to right across any period of the periodic table. The force increases as the nuclear charge increases, decreases as the electron moves farther from the nucleus.
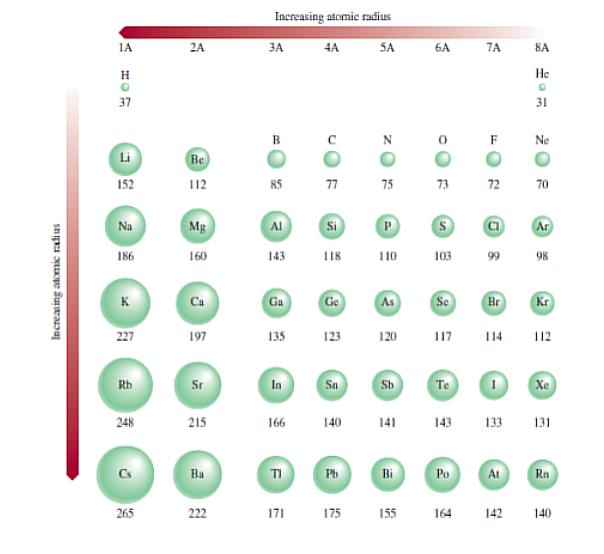
Atomic Radius
Left to Right = Decreases, Top to Bottom = Increases.
The size of an atom in terms of its atomic radius, which is one-half the distance between the two nuclei in two adjacent metal atoms or in a diatomic molecule
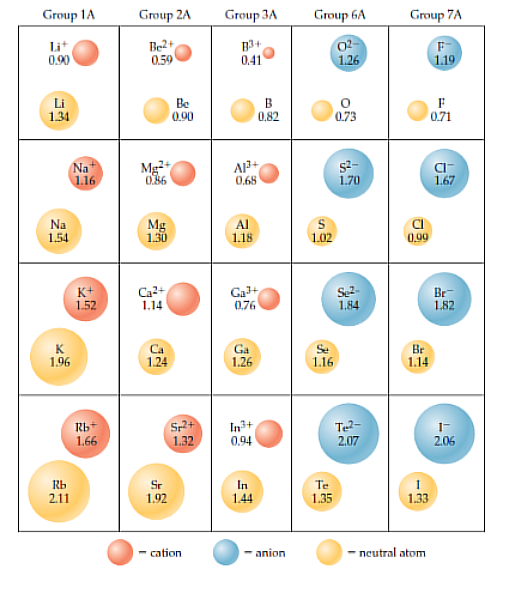
Ionic Radius
Ionic radius is the radius of a cation or an anion. Cations are smaller than their parent atoms. Anions are larger than their parent atoms.
Isoelectronic Series
Is a group of ions all containing the same number of electrons In any isoelectronic series, the more positive the charge, the smaller the ion.
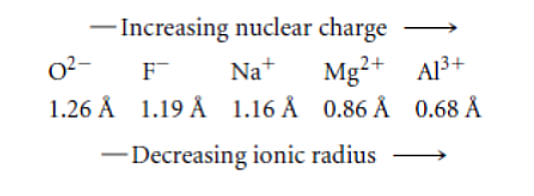
Ionization Energy
Left to right = increases, Top to bottom = decreases
The higher the ionization energy, the more difficult it is to remove an electron. Smaller atoms have higher ionization energy values
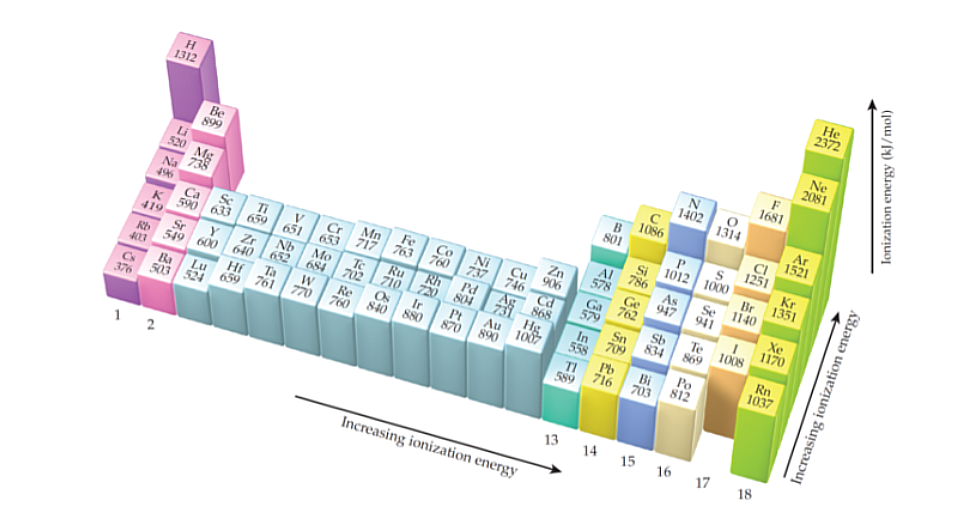
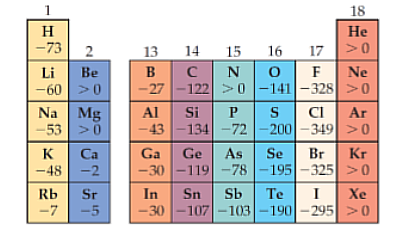
Electron Affinity
Left to right = increases, Top to bottom = decreases
Measures the energy change when an atom gains an electron. Where Elements with high electronegativity have a greater tendency to attract electrons.
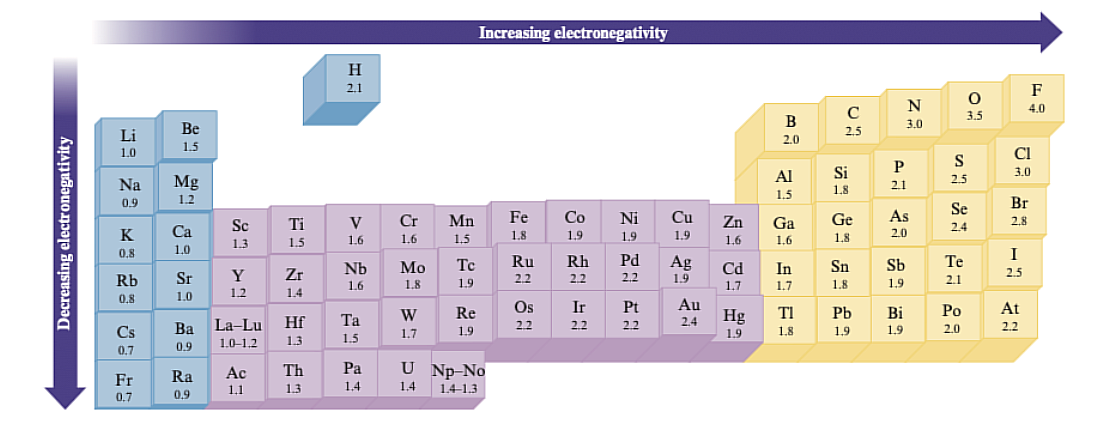
Electronegativity
Left to right = increases, Top to bottom = decreases
The ability of an atom in a molecule to attract shared electrons to itself. Elements with high electronegativity have a greater tendency to attract electrons.
G. N. Lewis
(1875–1946), An American Chemist that suggested a simple way of showing the valence electrons in an atom and tracking them during bond formation.
Lewis Symbol
The symbol created by G. N. Lewis. The dots are placed on the four sides of the Symbol (top, bottom, left, and right). Each side can accommodate up to two electrons. All four sides are equivalent, which means that the choice of sides for placement of two electrons rather than one electron is arbitrary. (The image shows 6 dots = 6 electrons).
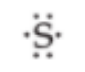
Octet Rule
This Rule explains that during chemical bonding. Atoms often gain, lose, or share electrons to achieve the same number of electrons as the noble gas closest to them in the periodic table (Usually Eight Valence Electrons).
Ionic Bonding
Metal (Cation) And Non-Metal (Anion) Bond together. By losing those electrons, these metals can achieve a noble gas configuration and satisfy the octet rule. Similarly, nonmetals that have close to 8 electrons in their valence shell tend to readily accept electrons to achieve their noble gas configuration (octet rule).
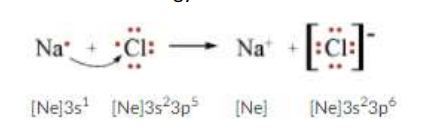
Structure of Ionic Compounds
Ionic Compounds form when A Metal and Non-Metal react. Metals are relatively stable if it loses electrons to form a complete valence shell and becomes positively charged. While A non-metal becomes stable by gaining electrons to complete its valence shell and become negatively charged.
Melting Points
Is a part of Properties of Ionic Compounds. This Physical Property states that Because of the many simultaneous attractions between cations and anions that occur, ionic crystal lattices are very strong. The process of melting an ionic compound requires the addition of large amounts of energy in order to break all of the ionic bonds in the crystal. For example, sodium chloride has a melting temperature of about 800oC. As a comparison, the molecular compound water melts at 0 °C.
Shattering
Is a part of Properties of Ionic Compounds. This Physical Property is generally hard but it is brittle. The repulsive forces between like-charged ions cause the crystal to shatter.
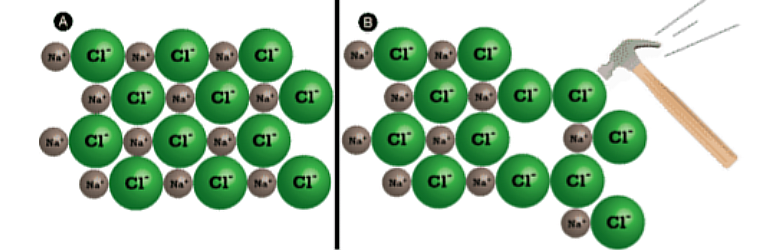
Conductivity
Is a part of Properties of Ionic Compounds. This Physical Property states that is that Ionic Compounds is their Electrical conductivity.