11.1 Organic Chemistry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
23 Terms
Name 3 features of homologous series
All memebers contain the same functional group. The functional gives that specific homologous series its chemical characteristics.
All members have the same general formula
Each subsequent compound in the series differes by a CH2 unit.
Hydrocarbon
A compound containing only Hydrogen and Carbon only.
Give 3 reasons why Carbon forms a large number of compounds.
Can form, single, double and triple bonds
Can form Carbon chains
Carbon forms bonds to other atoms such as Oxygen, Nitrogen and the Halogens.
What is the formula for Alkanes?
CnH2n+2
What is the formula for Alkenes?
CnH2n
What is the formula for Alkynes?
CnH2n-2
Formula for Alkyl group?
CnH2n+1
What is an Alkyl group?
A small fragment of the molecule
What is the formula for Alchohols?
CnH2n+1OH
What is the formula for Carboxylic acid?
CnH2n+1COOH (where n is the number of carbon atoms in the molecule, minus 1).
e.g Decanoic acid contains 10 carbons so n = 10-9 so the molecular formula will be C9H19COOH.
What is the other formula for carboxylic acids?
CnH2nO2
General formula for ketones?
CnH2nO
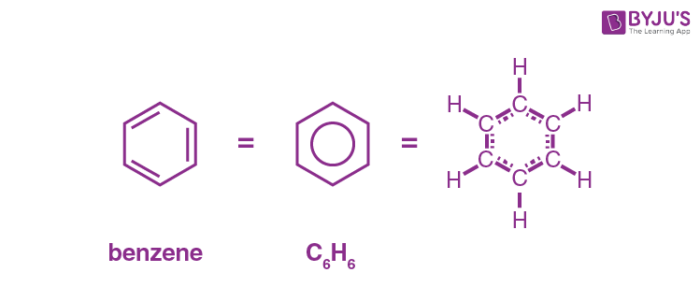
What does Aromatic skeleton mean?
Contains benzene rings
Benzene rings are hexagonal structures of carbon atoms with a ring of delocalised π-electrons.
What does Aliphatic skeleton mean mean?
A compound containing Hydrogen and Carbon joined together in straight chains, branched chains
Alicyclic
an aliphatic compound arranged in non-aromatic rings with or without side chains
Saturated?
only C-C bonds
Unsaturated?
C=C, C≡C
Structural isomers?
Compounds with the same molecular formulae but different structural formulae in 3D arrangement.
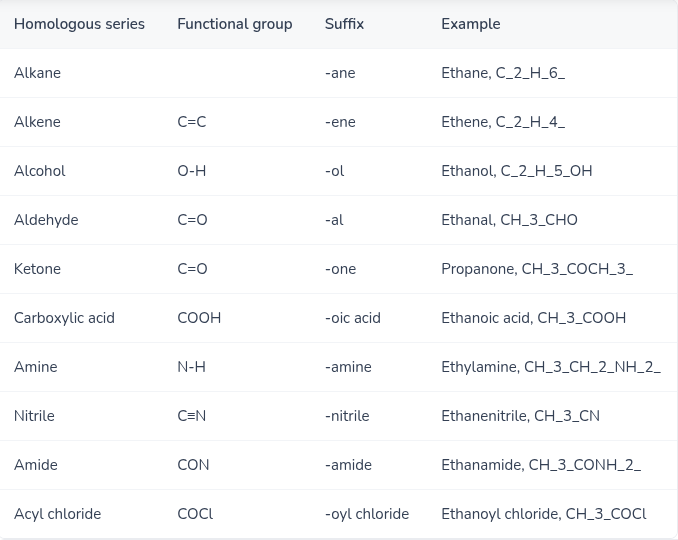
Give the suffix and functional group for each of the following:
Alkanes — Alkene — Alcohol — Aldehyde — Ketone — Carboxylic acid— Amine — Nitrile — Amide — Acyl chloride
-
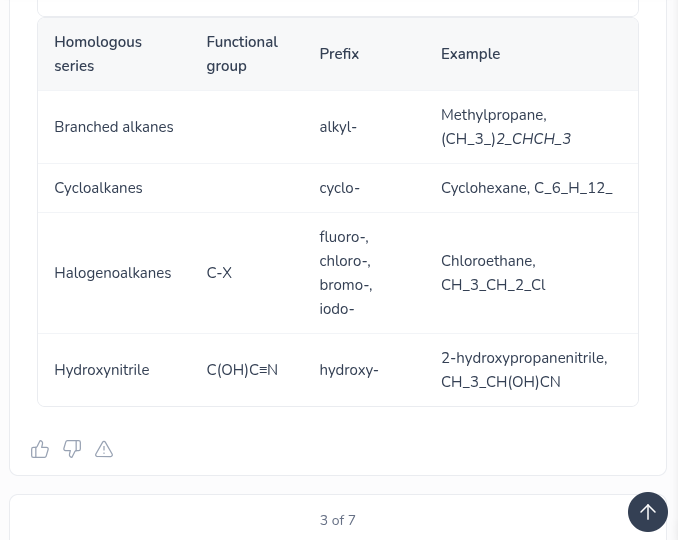
Give the prefix and functional group of the follwing:
Branched alkanes — Cycloalkanes — Halogenoalkanes — Hydroxynitrate
-
How to name an organic compound- general
Find the longest continous carbon chain- gives the stem name
Identify the functional group- gives suffix
Number the longest chain from the functional group (i.e the base chain)
Add side chain (alkyl groups) prefixes
Use multipliers if there are multiple identical groups
Put the name together- the sides chains are ordered alphabetically
How to name esters
Identify the alkyl group from the alcohol- the prefix
Identify the acid group and replace ‘-oic’ with ‘oate’ gives the second part of the name
Branched acids or alochols:
Number the α-carbon atom next to the ester functional group (COO) as carbon 1.
Name other substituents based on their position
How to name