11.2 dating fossils
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
what is dating?
determining the age of the material of fossils and artefacts.
what is absolute dating
the actual age (in years) of a fossil or artefact
what is the relative date
the age of a fossil or artefact relative to another fossil or artefact
what are the absolute dating methods
Radiometric (radioisotope or radioactive) dating is an absolute dating method.
what is radiometric dating
Determines the chronological age of a rock or mineral by measuring the proportions of an original radioactive material and its decay product.
what are radioisotopes
isotopes of the elements
• Radioisotopes are unstable and break down or decay to form more stable isotopes of another element (the daughter product).
• Radioisotopes are radioactive, emitting radiation as they undergo decay, which can be measured.
what is a half life
the time taken for half of any given amount of the radioactive isotope to decay
what are the radioisotopes commonly used for radiometric dating
• Carbon-14 (5730 years)→ Nitrogen-14
• Potassium-40 (1.3 billion years)→ Argon-40
• Rubidium-86 (18.66 days) → Strontium
• Uranium-238 (4.5 billion years) → Lead
potasium - argon dating
• Decay of radioactive potassium to form calcium & argon
• Decay takes place at a slow, constant rate
• Determining amounts of K-40 and Ar-40 can determine age of rock
• As rock ages proportion of K-40 decreases while Ar-40 increases
• To determine the age of a fossil using this method a suitable rock of the same age as the fossil must be available (age of fossil inferred by comparing to age of rock)
• Useful for bones buried during a volcanic eruption
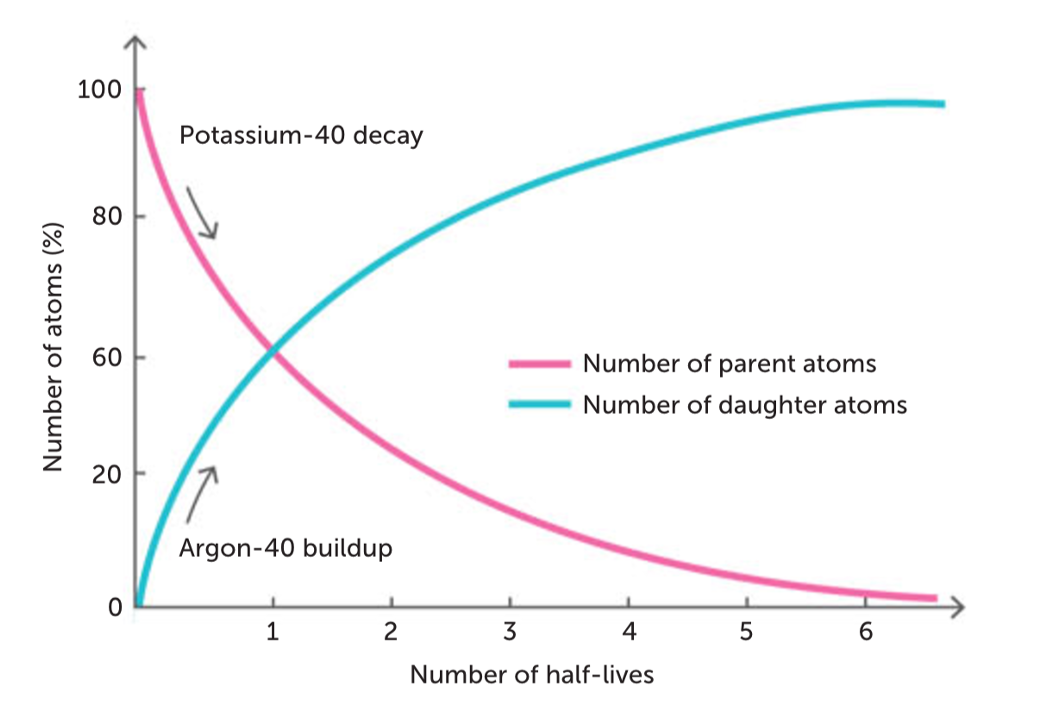
what are the limitations for potassium argon dating
• Not all rock types are suitable for this type of dating
• can only date volcanic rocks older than ~100,000 to 200,000 (most useful to date rocks older than 200,000 years)
• Half-life of 1250 billion years
• Takes 1250 billion years for half the K-40 to decay
• After 100 000 years only 0.0053% of K-40 would have decayed to Ar-40 – pushes the limit of detection devices we have
radio carbon/carbon-14 dating
• Decay of C-14 to N-14
• C-14 produced in upper atmosphere by action of cosmic radiation on nitrogen at the same rate that it decays
• In atmosphere ratio 1 C-14 atom per million million (1012) atoms of C-12
• Green plants use CO2 one atom in every 1012 is C-14
• Plant then eaten by animal \ becomes part of animal
•Death – intake of C-14 stops but decay of C-14 to N-14 continues at fixed rate
•Measure the ratio of C-14 to C-12 to work out the age
•Half life of 5730 years
method of radiocarbon dating
The normal method requires at least 3 grams of organic material
Accelerator Mass Spectrometry (AMS) radiocarbon dating can use an organic sample as small as 100 micrograms
Break the sample up into its constituent atoms & count the number of C-14 atoms
• Has been used to date cave paintings from pigments
what are the limitations of radiocarbon dating
• After 60,000 years amount of C-14 becomes negligible and unable to measure accurately
• Cannot use to date back more than 60 000 years
• Material must be organic (compounds from living things which contain carbon)
• Assumption that ratio of C-14:C-12 in the atmosphere was constant – we now know that it varies
• Corrections for fluctuations in C-14 content of atmosphere are possible for the past 9000 years by reference to other information such as tree-ring dating
what is it useful for
• Dating fossils of more recent origins
• Dating artefacts – often found in association with charcoal from cooking fires
• Date charcoal and can approximate the age of the artefact
what are relative dating techniques
Used to determine whether the fossil or artefact is older or younger than another sample or rock/soil it was found in
Allows for a sequence of events to be established eg. A is older than B.
Examples include:
•Superposition (for a single outcrop)
•Comparative stratigraphy (for 2 or more outcrops)
•Index fossils (for 2 or more outcrops)
•Fluoride dating
what is stratigraphy
the study of layers or strata.
two ways it can be useful in dating fossil material
principal of super position
correlation of rock strata
what is the principal of super position
assumes that in layers of sedimentary rock the top layers are younger than the ones below unless disuturbed
what are issues with it?
• It assumes that the fossils/artefacts are not disturbed
• Animals or early humans could have dug up/moved remains after deposition of sediments
• Distortions of the Earth’s crust occur
what is comparative stratigraphy or correlation of rock
• Matching layers of rock from different areas
• If the sequence of sedimentary rocks in different areas is similar it is likely that they are of the same age
• Done by examining the rock itself or by examining the fossils within the rock
• Rocks which contain the same fossils may be assumed to be the same age
what are index fossils
• Fossils of distinctive appearance, have a short time span and have a broad geographical distribution
• Provide information about the environment
• If rocks in different locations contain the same index fossils, it is likely that both areas are of the same age.
fossilised pollen grains importance
• Useful as index fossils
• Construction of type and amount of vegetation during that time period
• Climatic conditions may then be worked out.
• The data can then be used to refute or confirm relative dates arrived at by other methods
what are problems with the fossil record
the fossil record is very incomplete as conditions for fossilisation do not always occur, or occur at irregular periods of time
four conditions are usually required
quick burial of material
the presence of hard body parts
absence of decay organisms
a long period of stability - organism left untouched