BSCI201: LEx3
1/196
Earn XP
Description and Tags
Anatomy and Physiology 1 | Goytia and Fox
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
197 Terms
A muscle is considered an ______ because it is composed of muscle tissue, fibrous connective tissue (DRCT, DICT), nervous tissue, and blood tissue (vascularized and innervated). Muscle cells grow primarily by _____________.
organ; cellular hypertrophy
What are the special characteristics of muscles?
Contractility (can shorten), Excitability (transduces chemical signal to electrical signal), Extensibility (can extend), and Elasticity (returns to its original shape)
What is the hierarchical organization of the muscle organ starting from the contractile unit?
Sarcomere (contractile unit) → Myofibril (proteins) → Myofiber (cell) → Fascicle (tissue) → Muscle (organ)
What are the three Connective Tissue (CT) Sheaths?
Epimysium, Perimysium, and Endomysium
What layer is the Epimysium? What does it wrap?
The most superficial layer, wraps the entire muscle organ
What layer is the Perimysium? What does it wrap? What tissue is it composed of?
The middle layer; wraps a fascicle; contains areolar and dense irregular CT
What layer is the Endomysium? What does it wrap? What tissue is it composed of?
The deepest layer; wraps each individual myofiber; composed of areolar CT
What are the components of the sarcomere?
Z discs, I band, A band, H zone, M line
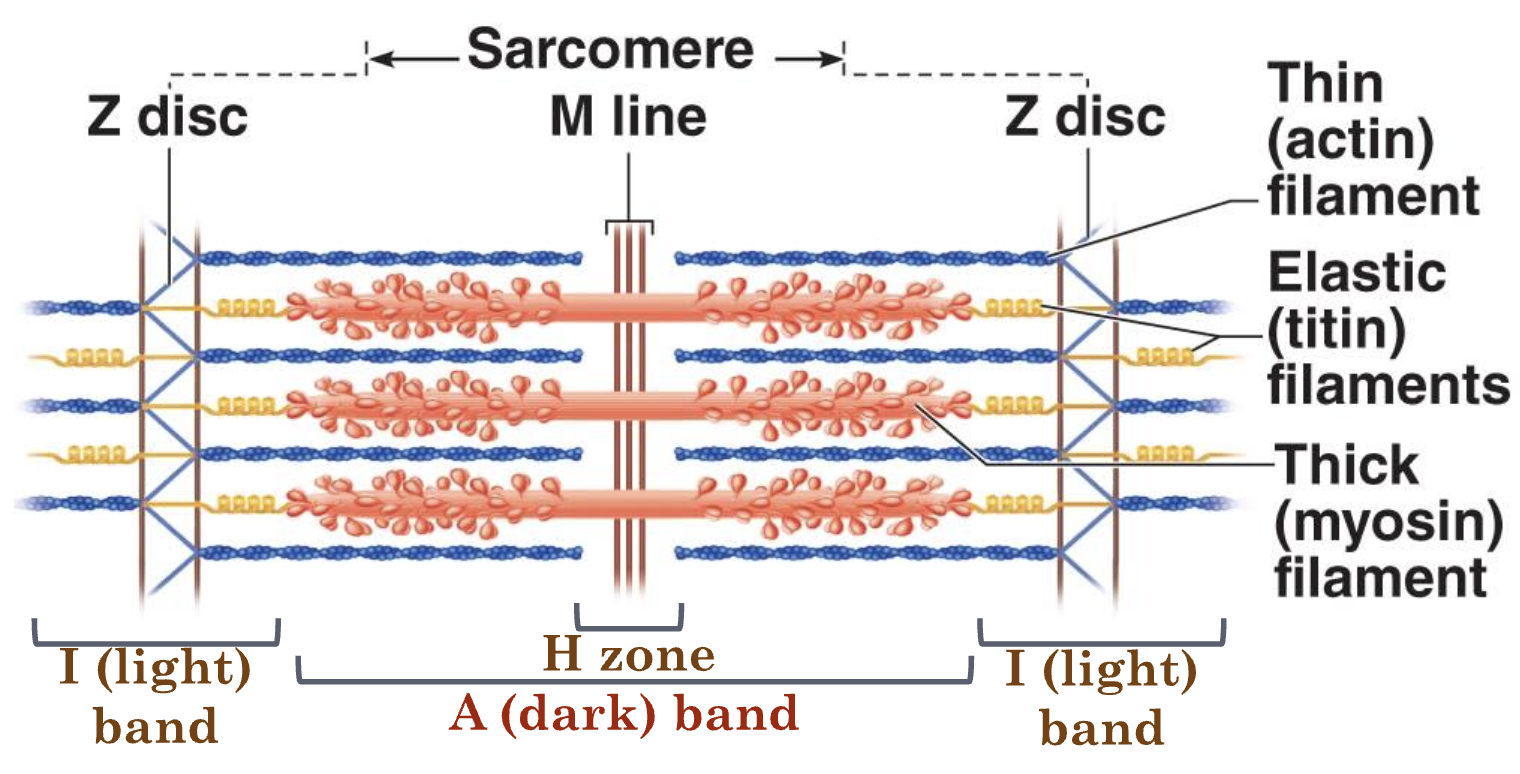
What is this mnemonic for: Ziggy Is An Honorable Man
Z disc, I band, A band, H zone, M line
What are the contractile proteins in the sarcomere? What is its function in contraction?
Myosin (thick filament) and Actin (thin filament); perform the activity of shortening the sarcomere
What are the regulatory proteins of the sarcomere? What is its function in contraction?
Troponin and Tropomyosin; regulate the activity of the contractile proteins
What are the structural proteins of the sarcomere? What is its function in contraction?
Titin, Z-disc proteins, M-line proteins; maintain the sarcomere’s structure
Myosin
Thick Filament
Actin
Thin Filament
What do the Z discs do?
Anchors thick (actin) filaments
What are the I bands? What happens to them during contraction?
Contains only thick filaments; shortens during contraction
Do A bands change during contraction?
Does not change length during contraction
What happens to H zone during contraction?
Shortens/disappears during contraction
Titin
connects myosin to Z disc and M line (“tug-of-war”)
What is the sliding filament theory?
Contraction occurs when myosin heads bind to actin (cross-bridge) and execute a power stroke, causing the thin filaments to slide inward past the thick filaments, shortening the sarcomere.
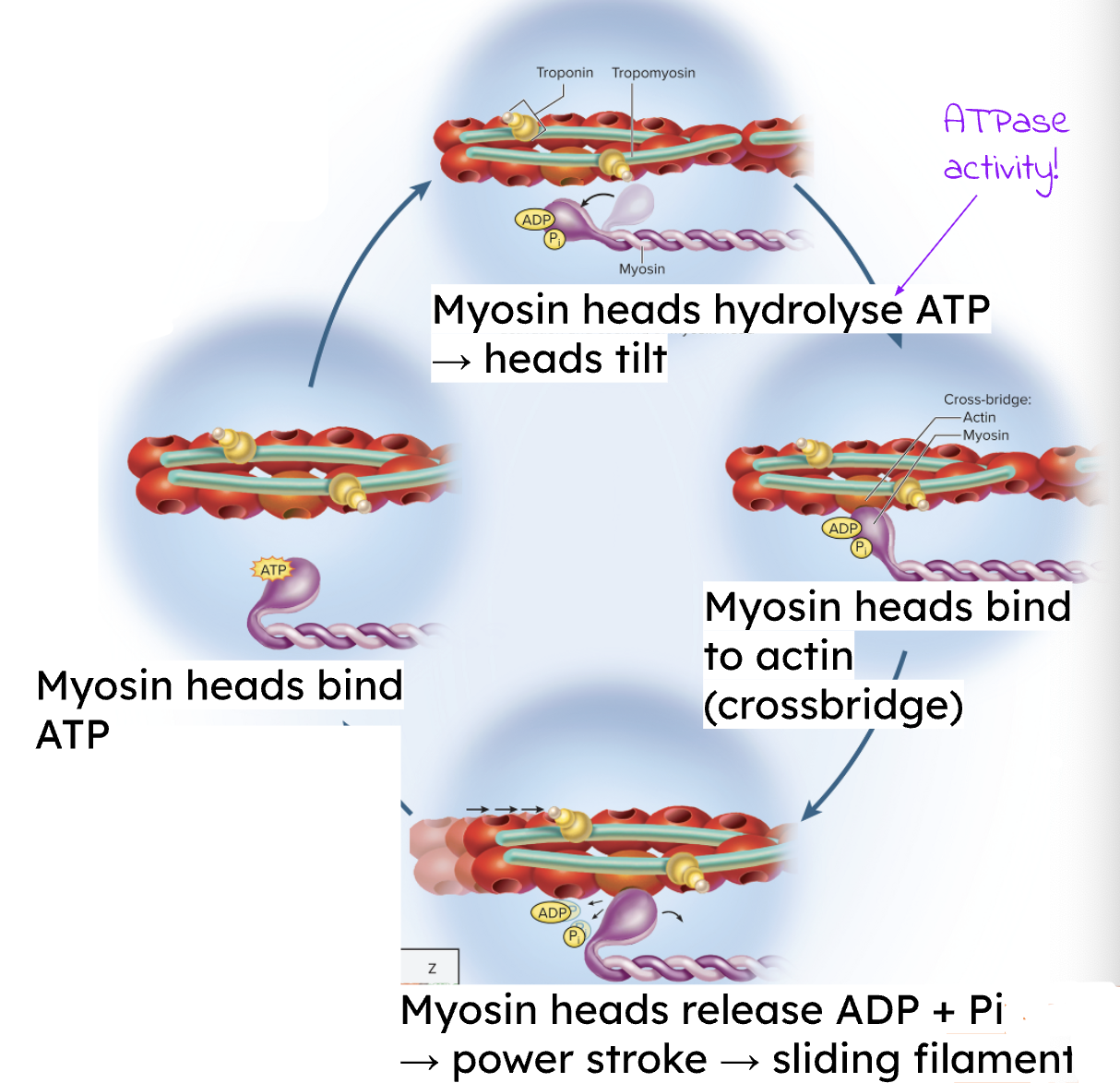
What is the role of Ca2+ in the sarcomere?
Ca2+ binds to troponin → causes a conformational change → shifts tropomyosin → unmasks the myosin-binding sites on actin, allowing cross-bridge formation
What is the role of ATP to the sarcomere?
Required for three main actions:
1) To activate (tilt) the myosin heads prior to cross-bridge attachment
2) For cross-bridge detachment (releasing myosin from actin)
3) For sequestration of Ca2+ back into the Sarcoplasmic Reticulum (SR)
At the neuromuscular junction (MNJ), the membrane of the muscle cell is the ___________________.
Postsynaptic cell membrane
The enzyme present in the neuromuscular junction’s (NMJ) synaptic cleft that degrades the neurotransmitter is the ______________.
Acetylcholine esterase
What is the Excitation-Contraction (EC) Coupling?
Process where muscle cell excitation (Action Potential) leads to contraction.
Involves the Neuromuscular Junction (NMJ) where Acetylcholine (ACh) is released by a motor neuron.
What are the 3 steps to the Excitation-Contraction (EC) Coupling and Contraction Cycle?
Excitation at the Neuromuscular Junction (NMJ)
Coupling and Ca2+ Release
Contraction (Sliding Filament Theory
Excitation at the Neuromuscular Junction (NMJ)
A nerve impulse (action potential, AP) arrives at the axon terminal of the motor neuron.
This triggers the release of the neurotransmitter acetylcholine (ACh) into the synaptic cleft.
ACh binds to ligand-gated ion channels (Na + /K + channels) on the sarcolemma (muscle cell membrane).
Ion influx (primarily Na + entering the cell) causes depolarization (electrical change)
Coupling and Ca2+ Release
The wave of depolarization spreads along the sarcolemma and travels deep into the fiber via T-tubules (invaginations of the sarcolemma).
At the triad (T-tubule plus two terminal cisternae of the SR), the electrical change stimulates voltage-gated receptors.
This stimulation causes the terminal cisternae of the Sarcoplasmic Reticulum (SR)—which stores Ca2+—to release stored Ca2+ into the sarcoplasm (cytosol)
Contraction (Sliding Filament Theory)
Ca2+ binds to troponin.
This binding causes a conformation change in troponin, which pulls tropomyosin away from the myosin-binding sites on the actin thin filament, unmasking them.
If ATP is present, the myosin heads (which are tilted by prior ATP hydrolysis) bind to actin, forming a cross-bridge.
The myosin heads release ADP and Pi, initiating the power stroke that pulls the thin filaments toward the M line, shortening the sarcomere.
A new ATP molecule binds to the myosin head, causing the cross-bridge to detach from the actin. The cycle continues as long as Ca2+ and ATP are available
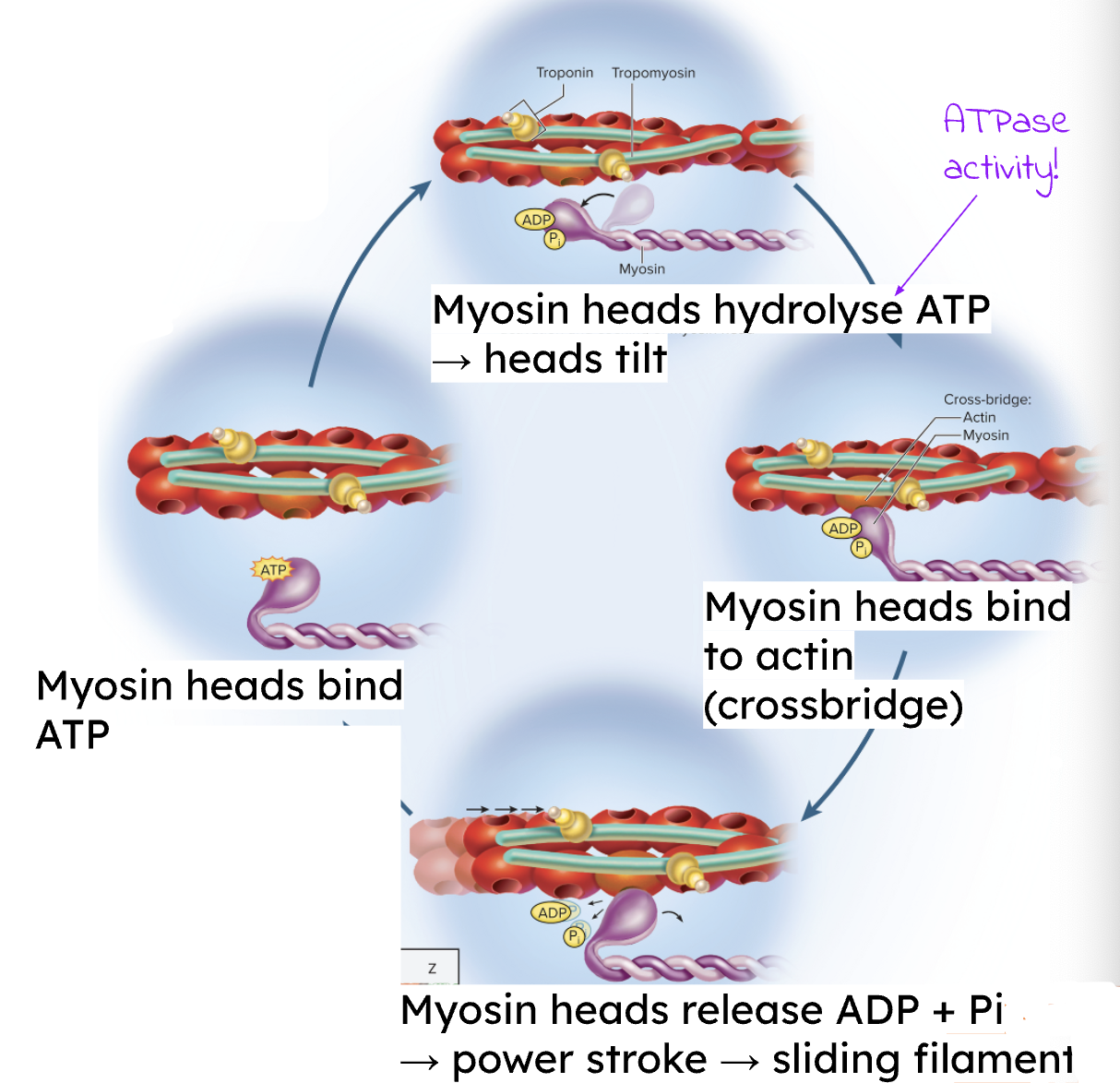
What are the steps to Muscle Relaxation?
Stop ACh release from the axon terminal.
Degrade ACh in the synaptic cleft using ACh Esterase.
Close ligand-gated ion channels on the postsynaptic membrane, allowing the membrane potential to return to resting state.
Sequester Ca2+ back into the SR (requires ATP).
Tropomyosin re-blocks the myosin-binding sites on actin.
Elastic structural proteins (Titin) reshape the sarcomere.
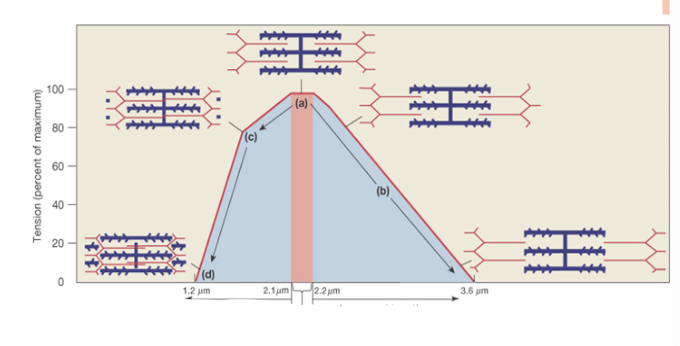
What is the Length-Tension Curve? Where is the optimal length?
Tension (force) generated is directly proportional to the number of cross-bridges formed. Optimal (resting) length (2.1-2.2 μm) produces maximum force because all myosin heads overlap with thin filaments
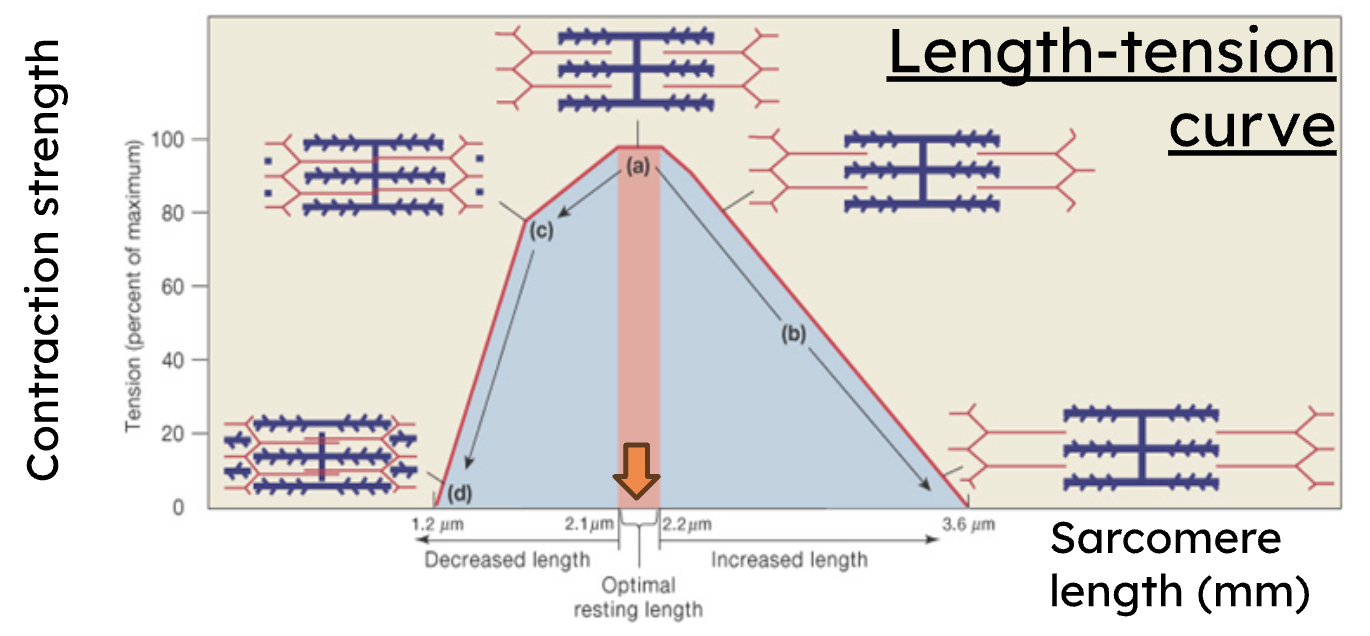
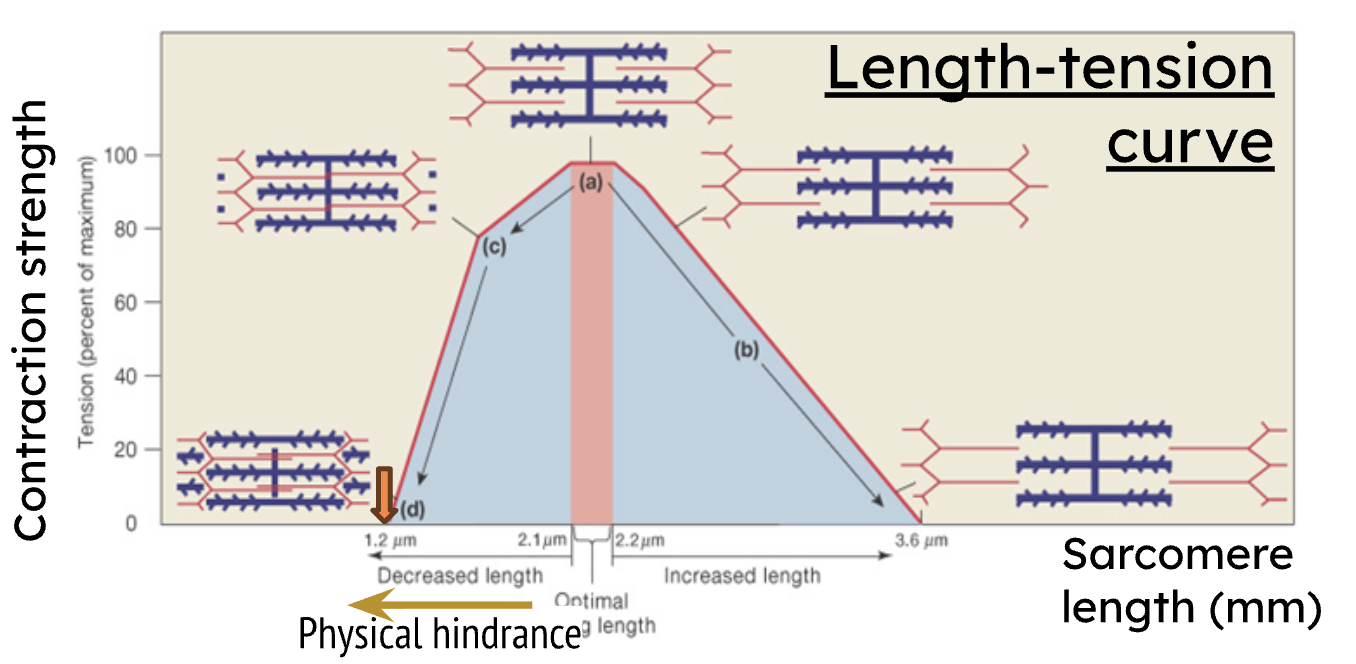
Decreased length on the Length-Tension Curve results in what?
decreased force due to physical hindrance
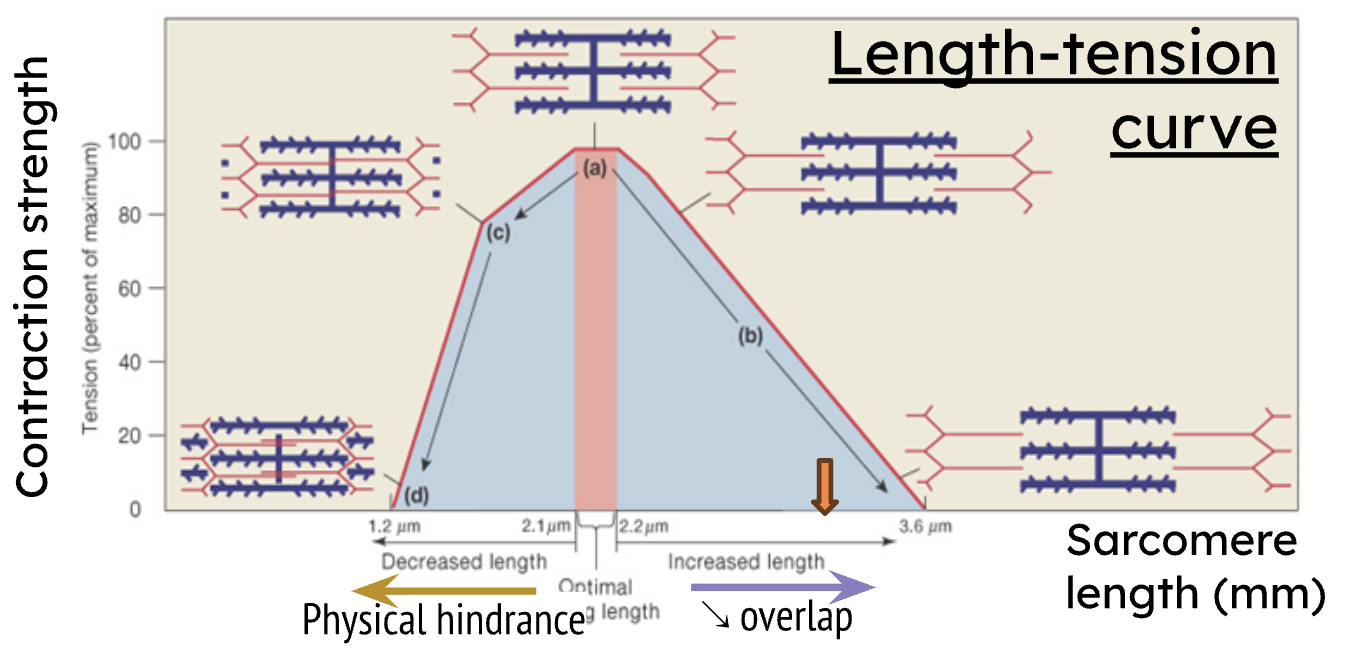
Increased length on the Length-Tension Curve results in what?
decreased force due to less overlap
What is a motor unit?
A motor unit is one motor neuron and all the muscle fibers it innervates.
Small motor units are for what?
fine movements (ie. fingers, eye muscles, etc.)
Large motor units are for what?
weight-bearing movements (ie. thighs, back, etc.)
What are the 3 major skeletal muscle types?
Slow Oxidative Fibers (SO), Fast Oxidative-Glycolytic Fibers (FOG), and Fast Glycolytic fibers (FG)
Fibers in a single motor unit are (different/same) type (SO, FOG, or FG).
same type
Slow Oxidative (SO)
Uses aerobic respiration (oxidative), highly fatigue-resistant, slow Myosin ATPase, high myoglobin/mitochondria, for sustained weight-bearing + posture
Fast oxidative/glycolytic (FOG)
Fast Myosin ATPase activity, moderately fatigue-resistant, ability to generate ATP using both aerobic (oxidative) and anaerobic (glycolytic) pathways
Fast glycolytic (FG)
Uses anaerobic glycolysis, easily fatigued, fast Myosin ATPase, large glycogen stores, low myoglobin/mitochondria
What process converts Fast Glycolytic muscle fibers into Fast Oxidative-Glycolytic fibers?
Endurance Training
Rigor Mortis
Stiffening of the body hours after death (3-6 hours post-mortem).
Occurs because muscle cells run out of ATP, preventing the myosin heads from detaching from actin (cross-bridge detachment) and preventing Ca2+ sequestration, leading to sustained, involuntary contraction.
Skeletal muscle tissue is attached to what? What characteristics does it have? Is it voluntary or involuntary?
Attached to bones and skin; striated and multinucleated; voluntary
Cardiac muscle tissue is found where? What characteristics does it have? Is it voluntary or involuntary?
Heart; striated (sarcomeres) and intercalated disks, uni/bi nucleated; involuntary
Smooth muscle tissue is found where? What characteristics does it have? Is it voluntary or involuntary?
Walls of hollow organs (stomach, urinary bladder, and airways); spindle-shaped cells, not striated (no sarcomeres), uninucleated; involuntary
How is the regeneration for Skeletal muscle?
minimal division (mostly hypertrophy via satellite cells)
How is the regeneration for Smooth muscle?
High capacity for repair/regeneration
How is the regeneration for Cardiac muscle?
Minimal cell division after infancy (mostly hypertrophy)
What is the Ca2+ regulator for skeletal muscle?
Troponin
What is the Ca2+ regulator for smooth muscle?
Calmodulin
What is the Ca2+ regulator for cardiac muscle?
Troponin
What is the contraction initiation for skeletal muscle?
Ca2+ → Troponin → Tropomyosin shift → Cross-bridge
What is the contraction initiation for smooth muscle?
Ca2+ → Calmodulin → Activates MLCK→ Activates/Phosphorylates Myosin → Cross-bridge. Features latch phenomenon (sustained contraction with less ATP)
What is the contraction initiation for cardiac muscle?
Ca2+ → Troponin → Tropomyosin shift → Cross-bridge
Isometric contractions
Tension is generated, but the muscle length stays the same
Isotonic contractions
Muscle length changes
What are the two different isotonic contractions?
Concentric and Eccentric
Isotonic concentric
Muscle shortens and does work, shortening of sarcomeres
Isotonic eccentric
Muscle contracts as it lengthens
What impact will a Voltage-gated Na+ Channel Blocker (ie. Lidocaine) have on muscle contraction?
Blocks AP conduction, prevents excitation → Flaccid paralysis (no contraction)
What impact will an Irreversible ACh Agonist (activates receptor) (ie. Anatoxin-A) have on muscle contraction?
Irreversibly binds ACh receptors at NMJ, causing constant message for depolarization → Instantaneous and sustained contraction (→ rigor)
What impact will an ACh Esterase Inhibitor (ie. Sarin) have on muscle contraction?
Prevents ACh breakdown, leading to constant stimulation of receptors → Sustained contraction and loss of control of breathing muscles (→ asphyxia)
Drug keeps mechanical gate for chloride open, what happens?
Chloride will go into the cell, causing hyperpolarization → low chance for action potential
What are the anatomical differences between CNS and PNS?
CNS - brain and spinal cord, contained in dorsal body cavity surround by meninges + protected by bone
PNS - neural structures outside CNS (cranial nerves, spinal nerves, and sensory receptors), many subdivisions
What are the CNS Subdivisions?
Gray Matter (nuclei, cell bodies). White Matter (tracts, myelinated axons)
What is CNS functional role?
Integration and control center
What are the two different PNS?
Somatic Nervous System and Autonomic Nervous system
What is the physiological difference between somatic NS and autonomic NS?
Somatic NS - voluntary movement (skeletal muscle)
Autonomic NS - involuntary control (cardiac/smooth muscle, glands)
What is the PNS functional role?
Connects the CNS to the body periphery
What are the Autonomic NS subdivisions?
Sympathetic NS and Parasympathetic NS
What is the functional role of ANS?
Homeostasis control of internal organs
Parasympathetic Nervous System (PSNS) common name and goal
“Rest & Digest,” conserves energy and promotes maintenance functions
Sympathetic Nervous System (SyNS) common name and goal
“Fight or Flight,” prepares the body for activity or stress
SyNS and PSNS effect on the heart
SyNS increases heart rate while PSNS decreases heart rate
SyNS and PSNS regulation example: Urination
SyNS is active when “holding” urine
PSNS is active when “peeing”
Where are Afferent nerve fibers from? What do they do?
From PNS, generate signal

Where are Efferent nerve fibers from? What do they do?
From PNS, conduct signals

What are the 2 principle cell types?
Neurons and Neuroglia
Neuron
excitable cells (excitability) that transmit electrical signals (conductivity), and secrete neurotransmitters (secretion)
Neuroglia (glial cells)
supporting cells that hold and insulate neurons in place; supply nutrients and oxygen as well as eliminate waste
Do neurons divide?
generally amitotic (no cell division after birth) due to lack of centrioles (essential for chromosome distribution in mitosis) so no they do not divide
Dendrites
Receive incoming information (EPSP/IPSP) “ears”
Soma (Cell body)
Contains nucleus
Axon Hillock (Initial Segment)
Computes information; site of AP generation (high density of voltage-gated channels)
Axon
Transmits Action Potential (AP)
Axon Terminals/Synaptic End Bulb
Transduces electrical signal to chemical signal (NT release) “mouth”
Multipolar
Most common (>99% CNS neurons), many processes (one axon, ≥ two dendrites)
Bipolar
Two processes (one axon, one dendrite); uncommon (olfactory epithelium, retina)
Pseudo-unipolar
Single process splits into peripheral (dendrite) and central (axon) processes; exclusively sensory neurons; cell bodies found in ganglia.
What are the glial cells in CNS?
Astrocytes, Oligodendrocytes, Microglia, Ependymal Cells
Astrocytes
Form blood brain barrier, structural support, regulate brain function, regulate nutrients
Microglia
Macrophages to engulf/destroy pathogens and cell debris
Ependymal cells
Ciliated columnar cells which line the ventricles and circulate cerebrospinal fluid
Oligodendrocytes
Produce myelin for CNS axons (myelinates multiple axons)
What are the glial cells in PNS?
Schwann cells, Satellite cells
Schwann cells
Produce myelin for PNS axons (each Schwann cell wraps one segment of one axon)
Satellite cells
Support neuron cell bodies in PNS ganglia.
Where is the Myelin Sheath? What does it do?
segmented protein-lipid sheath around axon of nerve cell; protect against trauma, insulate axon, increases speed of nerve impulse transmission (→ saltatory conduction)
What is the term for CNS that is defined as bundle of axons?
Tract