Chem I Final Review
1/267
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
268 Terms
Ionic Compound
Compound where a metal reacts with a nonmetal
Polar Covalent Bond
Unequal sharing of electrons
If Electronegativity = Zero
Bond is covalent
If Electronegativity = Intermediate
Bond is polar covalent
If Electronegativity = Large
Bond is ionic
Dipole Moment
When molecules have a center of positive charge and a center of negative charge
aka when, in a compound, one element has a higher electronegativity than the other
Ion size determines…
Electronegativity increases…
Right way
Electronegativity decreases…
Down way
Cation formation occurs…
On left of periodic table
electrons occupy high energy orbitals, so can lose electrons high energy electrons but not low —> limits size of positive charge on cations
Anion formation occurs…
On right of periodic table
electrons occupy low energy orbitals
To create cations…
For main group elements, take electron from highest energy orbital
For transition elements, take uppermost s subshell first before valence d electrons
Cations Size
Smaller than atom formed from
Anions Size
Larger than atom formed from
Bond breaking is a process of
Endothermic process
Bond formation is a process of
Exothermic process
Calculating ΔH for a given reaction
= energy required to break bonds – energy released when bonds form
Calculating Formal Charge
Atom’s group # - [Amount of lone pair electrons + ½ amount of bonding pair electrons]
Electron-Pair Geometry: 2 groups around center
Linear
Bond angle: 180
Electron-Pair Geometry: 3 groups around center
Trigonal-planar
Bond angle: 120
Electron-Pair Geometry: 4 groups around center
Tetrahedral
Bond angle: 109.5
Electron-Pair Geometry: 5 groups around center
Trigonal-bipyramidal
Bond angle: 120 and 90
Electron-Pair Geometry: 6 groups around center
Octahedral
Bond angle: 90
If sum of lone pairs and atoms is 2, the hybridized orbital is…
Two sp orbitals
Unhybridized orbitals: Two p orbitals
If sum of lone pairs and atoms is 3, the hybridized orbital is…
Three sp² orbitals
Unhybridized orbitals: One p orbital
If sum of lone pairs and atoms is 4 the hybridized orbital is…
Four sp³ orbitals
Unhybridized orbitals: None
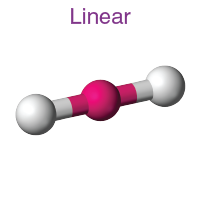
Geometry of sp hybrid orbital
Linear
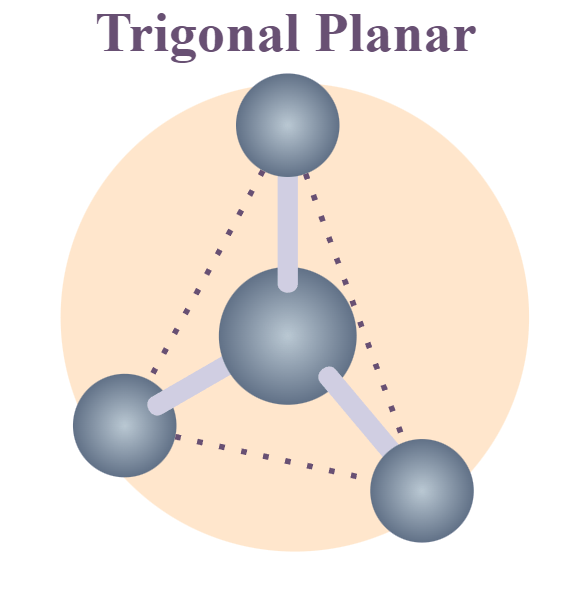
Geometry of sp² hybrid orbital
Planar-trigonal
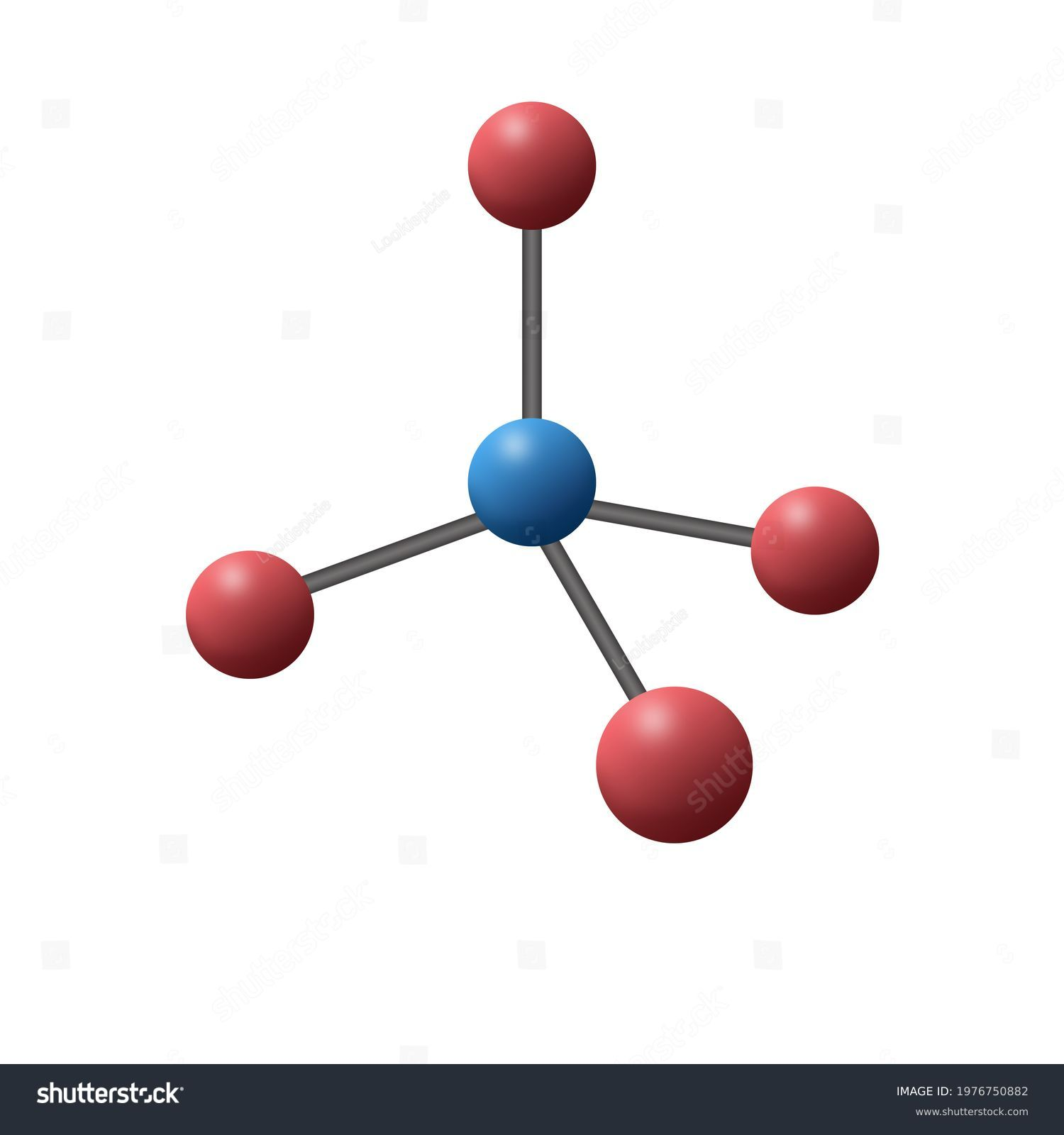
Geometry of sp³ hybrid orbital
Tetrahedral
When 1s orbitals combine…
They form two sigma molecular orbitals
one bonding, one antibonding
When 2p orbitals combine…
They do
Direct head-to-head to form sigma and sigma+ molecular orbitals
Indirect, parellel to form pi and pi+ molecular orbitals
Molecular Geometry of 3 atoms + 0 lone pairs
Trigonal Planar
Molecular Geometry of 2 atoms + 1 lone pairs
Bent
Molecular Geometry of 4 atoms + 0 lone pairs
Tetrahedral
Molecular Geometry of 3 atoms + 1 lone pairs
Trigonal Pyramidal
Molecular Geometry of 2 atoms + 2 lone pairs
Bent
Molecular Geometry of 6 atoms + 0 lone pairs
Octahedral
Molecular Geometry of 5 atoms + 1 lone pairs
Square pyramidal
Molecular Geometry of 4 atoms + 2 lone pairs
Square planar
Molecular Geometry of 5 atoms + 0 lone pairs
Trigonal bipyramidal
Molecular Geometry of 4 atoms + 1 lone pairs
Seesaw
Molecular Geometry of 3 atoms + 2 lone pairs
T-shaped
Most likely Resonance is…
The one with the smallest formal charge
Paramagnetism
Presence unpaired electrons
Diamagnetism
Presence of paired electrons
Bond order
(# of bonding electrons - # of antibonding electrons) / 2
Larger bond order
Greater bond strength
Steps for Drawing MO Orbital Energy Diagram
Think of it as combining two atoms of the same element: AtomA and AtomB
Draw out left to right the amount of electrons in the 1s orbital for the element on both sides
Draw the bonding sigma 1s orbital and antibonding sigma 1s orbital
Transfer electrons from both sides into bonding sigma 1s orbital first, then antibonding if needed
Repeat for sigma 2s, pi 2p, etc
Hydrogen Bonding
When hydrogen reacts with N, O, F
Wavelength (λ)
The distance between two peaks or troughs in a wave.
Electromagnetic Radiation
Radiant energy that exhibits wavelike behavior and travels at the speed of light.
Frequency (v)
The number of waves or cycles per given time period that passes a given point in space.
Photon
A stream of individual packets of energy associated with electromagnetic radiation.
Quantum
A packet of energy; energy is transferred only in whole multiples of hv.
Photoelectric Effect
The emission of electrons from a metal when light strikes it.
light must have a minimum frequency for electrons to be emitted
Threshold Frequency (Vo)
The minimum frequency required to remove an electron from a metal's surface.
Greater than threshold frequency = light emits
Lesser than threshold frequency = no light
Formula for Finding Kinetic Energy of an Electron
KEelectron = ½ mv² = hv -hvo
m - mass of electron
v - velocity of electron
hv - energy of incident photon
hvo - energy req to remove electron from metal’s surface
Bohr’s Theory of Hydrogen Atom
a) Has quantized energy levels
b) ELectrons only have certain allowed circular orbits
c) More tightly bound electron = more energy becomes negative
energy released from system as electron brought closer to nucleus
Limitation: Electrons don’t move in circular orbits
Orbital
A specific wave function that describes a region of space where an electron is likely to be found.
Heisenberg Uncertainty Principle
The more accurately we know an electron's position, the less accurately we can know its velocity, and vice versa.
Excited State
A state of an atom in which electrons have absorbed energy and moved to higher energy levels.
Quantized Energy Levels
Discrete energy levels that electrons can occupy within an atom.
Energy Change (ΔE)
The difference in energy between the final state and initial state of an atom.
Radial Probability Distribution
The measure of the likelihood of finding an electron in a particular region of space around the nucleus.
Degenerate Orbitals
Orbitals that have the same energy level.
Noble Gas Configuration
A shorthand notation that uses the last noble gas to denote electron configuration.
Waves Have Three Characteristics:
Wavelength, frequency, and speed
Long wavelength
Short frequency
Short wavelength
Long frequency
What is the relationship between wavelength and frequency?
They are inversely related.
Formula Relating Wavelength, Frequency, and Speed of Light
c = λv
c - speed of light (2.9979 × 108 m/s)
λ - wavelength (m)
v - frequency (cycles per second)
Converting wavelength from nm to m
Multiply by 10-9 m aka use conversion factor 10-9m / 1 nm
Calculating For Energy of a Photon
Ephoton = hv = hc / λ
h - Planck’s constant (6.626 × 10-34 J*s)]
v - frequency of radiation
λ - wavelength of radiation
Wavelength of Gamma Rays
10-12 m
Wavelength of X-rays
10-10 m
Wavelength of Ultraviolet
10-8 m
Wavelength of Visible Light
4 × 10-7 to 7 × 10-7 m
Wavelength of Infrared Light
10-4 m
Wavelength of Microwaves
10-2 m
Wavelength of Radio Waves
1 to 104 m
Relationship Between Energy and Wavelength
They are directly related.
Dual Nature of Light
Light can be a wave and photons
Calculating Wavelength for a Particle
de Broglie’s Equation
λ = h / mv
h in units of J*S or kg*m²/s
Look @ units given and determine units of h to cancel out other units
Check units of mass and frequency and determine if conversions are needed
Plug into eqn and cancel out units
Calculating Energy Required to Move Electron from a Level to Another
Note: Removing electron from its ground state means ninitial = 1 to nfinal = ∞
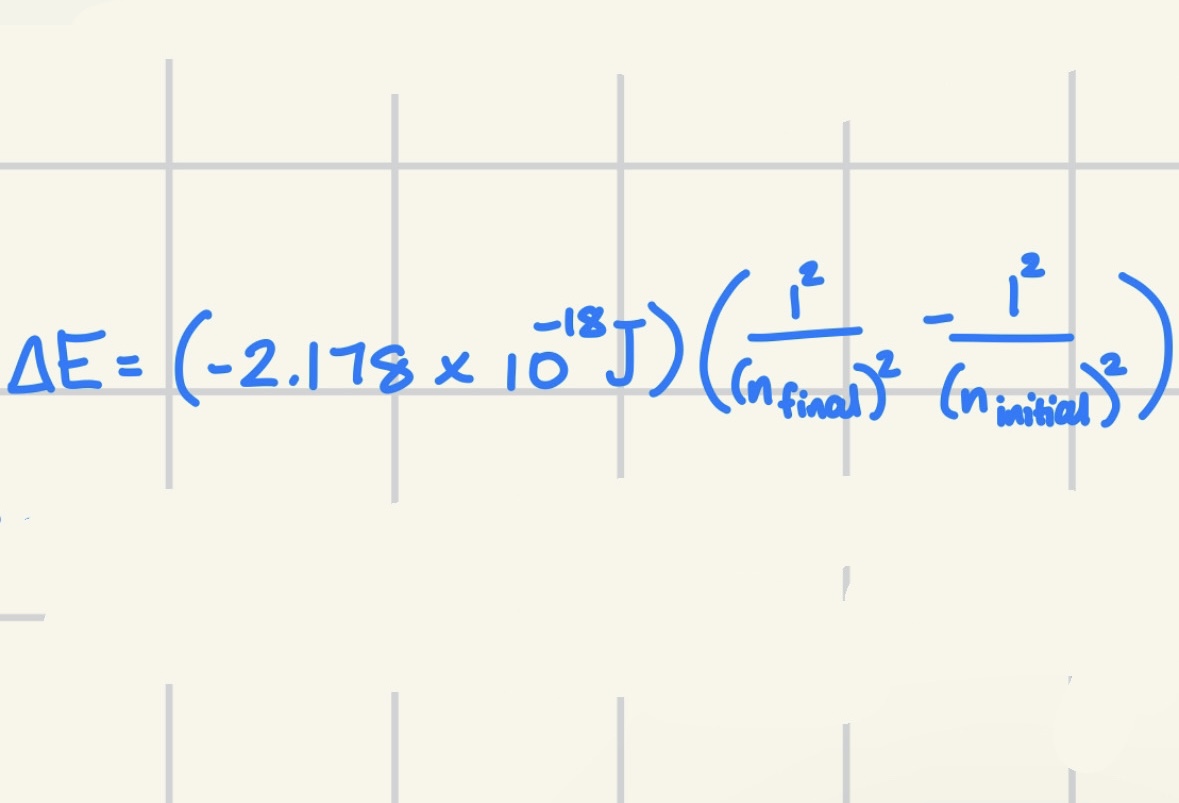
How to Calculate Wavelength of Emitted Photon
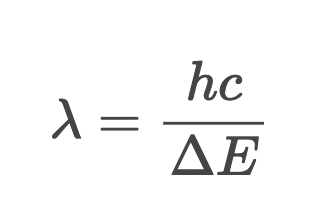
Quantum Model for Hydrogen Atom
A model where the electron is assumed to behave as a standing wave
electron moves in certain allowed circular orbits
assumes angular momentum of electron happens in certain increments
Quantum Numbers
Series of #s that describe properties of orbitals
Principal Quantum Numbers (n)
Tells energy level the electron occupies
As n increases:
Orbital becomes larger
Electron gets farther away from nucleus
Value of n correlates to # that goes before orbital sublevel letter (s, p, d, f)
Angular Momentum Quantum Number (ℓ)
Tells type of orbital we’re in
= 0→ s
= 1 → p
= 2 → d
= 3 → f
Has values from 0 to n-1
Magnetic Quantum Number (mℓ)
Tells the orientation of an orbital in relation to other orbitals in the same subshell
Has values from -ℓ to ℓ, including 0
Wavefunction Nodes (Nodal Surfaces)
Areas of zero probability in an orbital
# of nodes increases as n increases
p Orbital Shape
Dumbbell / peanut shape
ℓ = 1
d Orbital Shape
Clover leaf shape
ℓ = 2
s Orbital Shape
Spherical shape
as n increases, so does size
ℓ = 0
Number of Nodes for s Orbitals is Given by
n - 1
ℓ Angular Nodes
Nodes that cut through the nucleus
Radial Nodes
Nodes that circumscribe the nucleus
given by n - 1 - ℓ
Valence Electrons
Electrons present in outermost principal quantum level (n) of an atom
To find:
Identify largest n value (i.e. 4p6)
Count the amount of electrons in that specific level; these are valence electrons
Core Electrons
Inner electrons, aka not valence electrons
Periodic Trends of Atomic Radius
Decreases right way
Increases down way