Enzyme structure, specificity and mechanism
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
16 Terms
substrate binding - lock and key model
complimentary geometry and interactions between the enzyme and substrate(s) are needed for binding
substrate binding - induced fit model
enzymes are flexible proteins and able to change conformation, substrate binding often alters the shape of the enzyme by using the binding energy, optimal interactions with the enzyme occur only with the transition state.
Induced fit maximises the binding interactions with the transition state to reduce the activation energy required for the reaction to be accelerated
substrate binding - non-covalent interactions
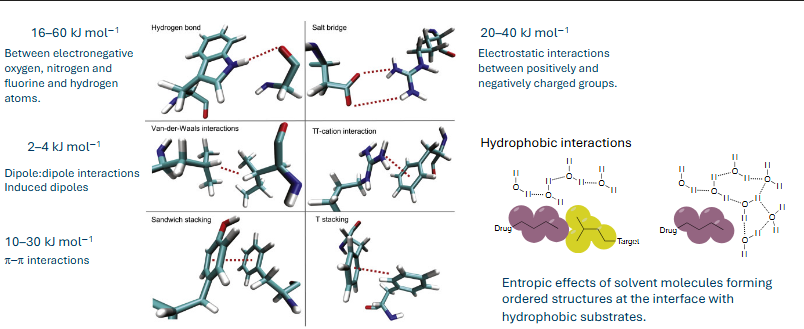
substrate binding interactions
antibiotic resistance to chloramphenicol is often the result of enzyme catalysed acetylation of the antibiotic which stops it from binding its target
serine proteases
digestive enzymes which hydrolyse peptide bonds to break down proteins and peptides
how do different serine proteases have different substrate selectivity
substrate binding pocket site close to the active site dictates selectivity
serine proteases - essential amino acids for catalysis
Biochemical identification of the essential catalytic serine was achieved using an organophosphorus compound to chemically label active site group.
Identifying essential amino acids in the structure and mechanism through sequence alignments
% identity - same amino acid
% similarity - amino acid with similar properties
serine proteases - catalytic mechanism
Catalytic triad: catalytic residues of Ser195, His57 and Asp102 form part of the active site pocket and are conserved across serine proteases.
3 major challenges
Stable peptide bond due to the resonance structure
Water is a poor nucleophile
The amine product is a poor leaving group
stabilisation of transition state
For serine proteases the tetrahedral oxyanion intermediate and transition state is stabilised through forming hydrogen bonds to the enzyme (oxyanion hole)
4 important concepts in serine protease catalysis
Catalytic triad increases the reactivity of the catalytic serine
The oxyanionic hole stabilises the intermediates and transition states
Covalent catalysis provides an effective mechanism to reduce the activation energy for catalysis
The covalent enzyme intermediates are hydrolysed to reform the active enzyme
preorganised active site electrostatics
Active site electrostatics preferentially stabilise the transition state changes which form transiently during the reaction.
general acid/base catalysis
Uses ionisable amino acids to provide or accept a proton as part pf the reaction cycle to accelerate catalysis
HAD (haloacid dehalogenases) phosphatases (hydrolases)
Magnesium (Mg2+) dependent enzymes
metal ion catalysis
Metalloenzymes - contain tightly bound metal ions. Fe2+/3+, Cu2+, Co3+, Zn2+
Metal-activated enzymes - loosely bound metal ions from solution often with the substrate, Mg2+, Ca2+, K+, Na+
Bind substrates and help orientate for catalysis
Mediate oxidation-reduction reactions
Electrostatic stabilisation and shield negative charge (acid/base)
Polarise bonds coordinating to the metal ion
Metallo-beta-lactamases are used by antibiotic resistant pathogenic bacteria to break down beta-lactam antibiotics
cytochrome p450 metalloenzymes
metal cofactors mediate oxidation-reduction reactions