M4L2 - Post-Translational Targeting to Mitochondria
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Characteristics of Mitochondria
1. Bound by a double membrane
2. Primary site of ATP production
Proteins of ETC are found in inner mitochondrial membrane
3. Has their own genomes
Mitochondrial genes code for a few of the proteins found in the mitochondira
4. They can reproduce by binary fission
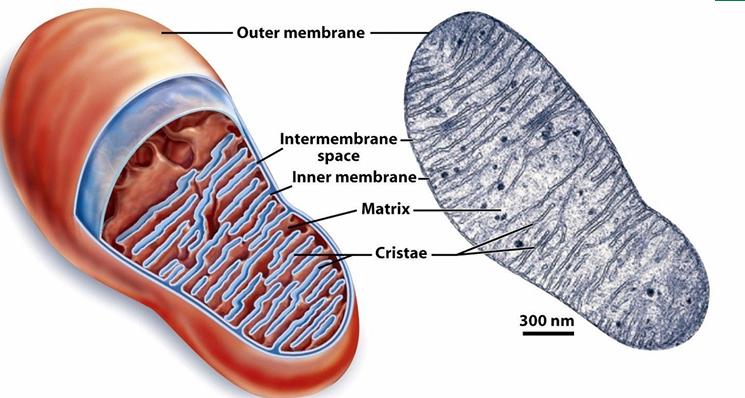
Internal Compartmentalization of Mitochondria
Has outer membrane
Has inner membrane with involutions to create cristae
Inc SA where ATP synthesis occurs from nutrients
This structure increases the capacity of mitochondria to create lots of ATP
Between membranes: Intermembrane space
Right: TEM image
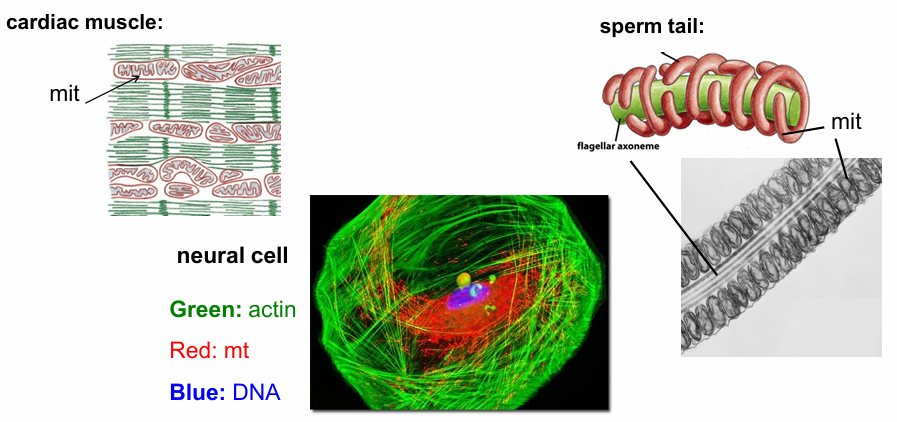
Mitochondria Distribution in Different Cell Types
Cells requiring a lot of ATP will have more mitochondria
Cardiac Muscle: (Top Left)
Actin bundles (green) required for continuous muscle cell contraction
Arrays of mitochondria present (red) throughout the cells
They constantly provide ATP to fuel cardian muscle contraction
Sperm Cell: (Top Right)
Moves rapidly, so needs a lot of E to maintain this movement
TEM image: Flagellar Axoneme Middle Cross-Section
Surrounding it is mitochondria
Forms mitochondrial tubules that wrap around the axoneme
Neural Cell: (Bottom Left)
Needs a lot of E to function
Nucleus (Blue labelled with DAPI)
Actin (Green labelled with phalloidin)
Mitochondria (Red labelled with antibody to mitochondrial-specific protein)
Mitochondria doesn’t show up at punctate dots but as a network of tubules
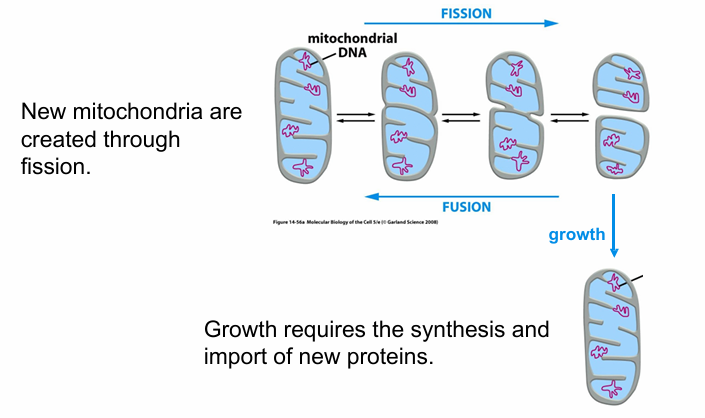
Mitochondria Cell Dynamics
Change shape
Undergo fission/fusion
Always moving around
Grows
Mitochondria Biogenesis
Requires protein synthesis
Contains much of its own genome
A single mitochondria can divide into 2 by fission
Each daughter usually ends up with one genome copy at least
if not, it would die
2 mitochondria can undergo fusion
Destinations of Proteins Within Mitochondria (4)
Outer membrane
Inner Membrane
intermembrane space
matrix of mitochondria
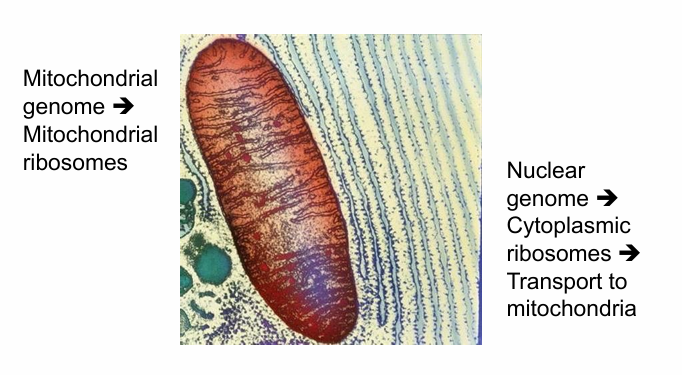
Where do Mitochondrial Proteins Come from?
Some are encoded in the mitochondrial genome
Synthesized using mitochondrial ribosomes
Majority are coded by nuclear genes
Genes transcribed in nucleas
mRNA translated in cytosol by free ribosomes
Proteins transported to mitochondria by pathway
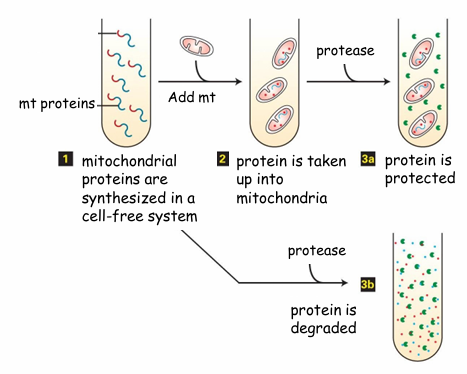
Post-Translational Protein Transport to Mitochondria Evidence
Hypothesis: If fully translated proteins can be transported, then proteins in the presence of mitochondria will move into it
Experiment:
Follow the protein synthesis in cell-free system
Tube 1: Polypeptides with mitochondrial signal sequence (red)
Experiment 1:
Add energized mitochondria first
Then add protease
Experiment 2: (Bottom)
Just add protease but no mitochondria
Result:
In experiment 1, the proteins are safe from degredation as they’re transported to mitochondria
In experiment 2, no mitochondria so proteins degrade
Conclusion: Fully translated proteins can be transported into mitochondria
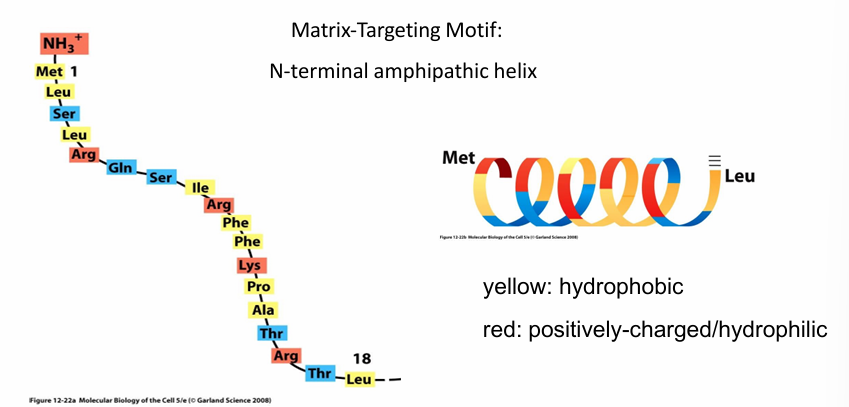
Rule 1: Protein Transport to Mitochondria
Matrix-Targeting Motif:
Peptide signal sequence
Found on N-terminus
18-50 amino acid long peptide
Forms an a-helix that is amphipathic
Red: (+) residues that are hydrophilic
Yellow: Hydrophobic residues
Regular arrangement of hydrophobic/philic residues allowing them to be on opposite surfaces when folded
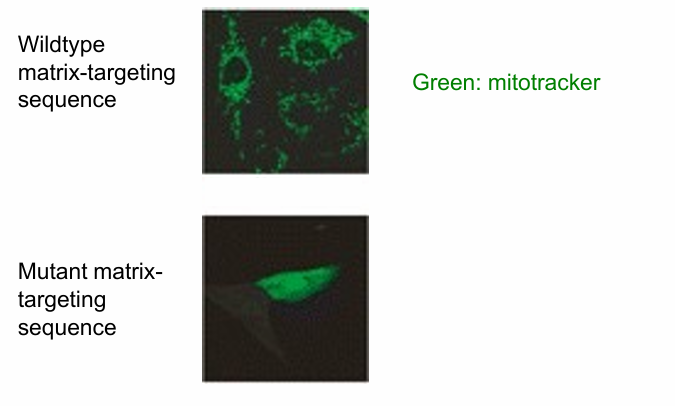
Is Matrix-Targeting Motif Necessary for Protein Transport to mitochondria?
hypothesis: if the amphipathic helix is disrupted, a mitochondrial protein will not go to the mitochondria
test by
creating mutations disrupting the amphipathic nature
eliminating the motif
Experiment:
Hydrophobic residues replaced by hydrophilic ones
Top Image: wild-type mitochondrial protein (unmodified matrix-targeting motf)
Present in mitochondria
Detected using antibody to the protein (green)
Bottom Image: Mutant matrix-targeting motif
mitochondrial protein remains in cytosol
Conclusion: Yes, matrix-targeting motif is necessary
Is Matrix-Targeting Motif Sufficient for Protein Transport to mitochondria?
Hypothesis: If matrix-targeting motif is added to GFP, then it will go to mitochondria
Result: The GFP with matrix-targeting motif went to the mitochondria as it was seen in a punctate pattern
Conclusion: Yes it is sufficient
Rule 2: Protein Transport to mitochondria
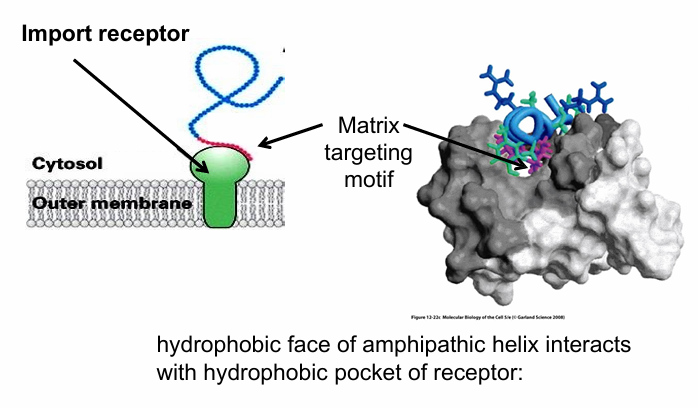
Import Receptor
A signal receptor recognizing matrix-targeting motif
Embedded in out membrane of mitochondria
How does it recognize the matrix-targeting motif?
Amphipathic helix of the motif fits into the hydrophobic binding pocket of the receptor
All hydrophobic residues of the amphipathic helix are facing the hydrophobic pocket
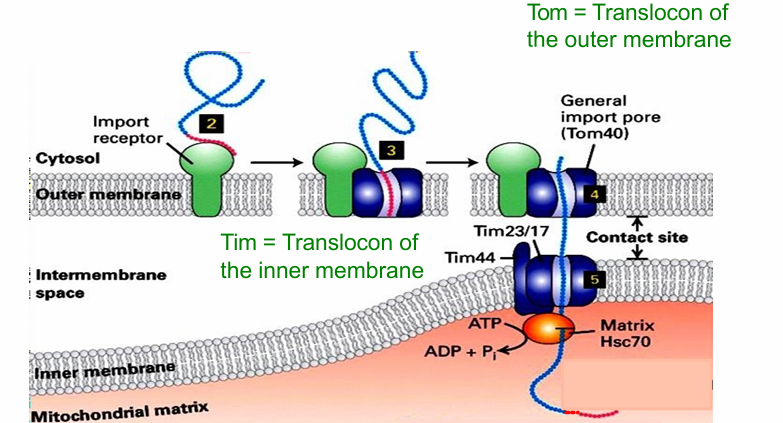
Rule 3: Protein Transport to mitochondria
General Import Pore (AKA Tom40)
Translocation channel
When bound to import receptor, the matrix-targeting sequence is translocated to the general import pore
The protein is shuttled through that translocation channel
If targeted to the matrix, it will continue into another translocon of the inner membrane
The Tim44, Tim23, Tim17 complex
At certain contact sites, the two translocons are aligned (Tim/Tom)
Allows for direct movement of protein going to matrix
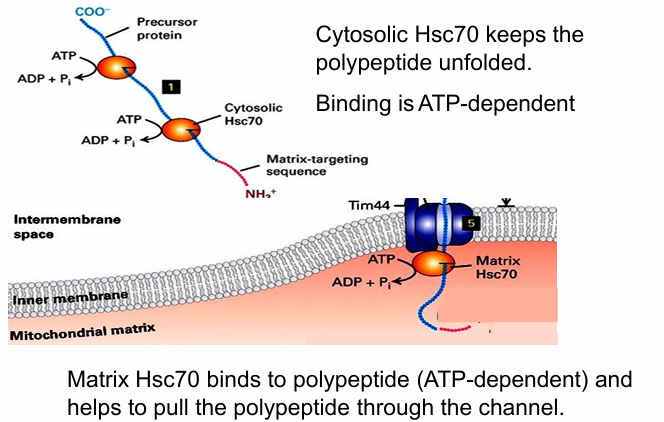
Rule 4: Protein Transport to mitochondria (2)
Instance 1:
Mitochondrial proteins first are translated in cytosol
They must remain unfolded to fit through Tom and Tim translocons
So they’re grabbed by cytosolic chaperone proteins (Hsc70) to keep them unfolded
Hsc functions requires ATP hydrolysis
Instance 2:
ATP hydrolysis needed in mitochondria during protein transport
Matrix Hsc70 grab unfolded protein as it enters matrix
Prevents the protein from moving backwards
ATP hydrolysis conformationally changes Hsc70 that will pull the protein into the matrix
Another Hsc70 comes to bind, enabling the full protein to be pulled through
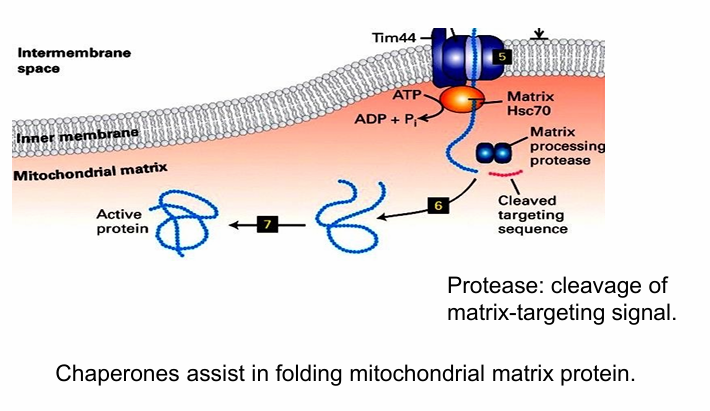
Protein Folding in Mitochondria (after transport)
Matrix processing protease removes N-terminal matrix-targeting sequence
Otherwise the protein won’t fold
Hsc70 proteins then further assist it in folding
Rule 5: Protein Transport to mitochondria
To transport to inner membrane there needs to be
N-terminal matrix targeting sequence
stop-transfer sequence
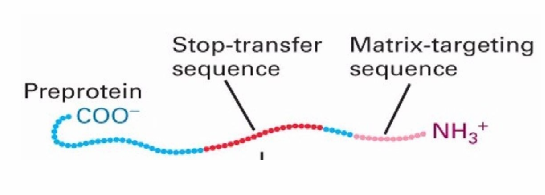
Stop-transfer Sequence
Hydrophobic sequence
found in the middle of the protein sequence
Helps the target protein go to the inner membrane as it gets stuck in lipid membrane bc hydrophobic
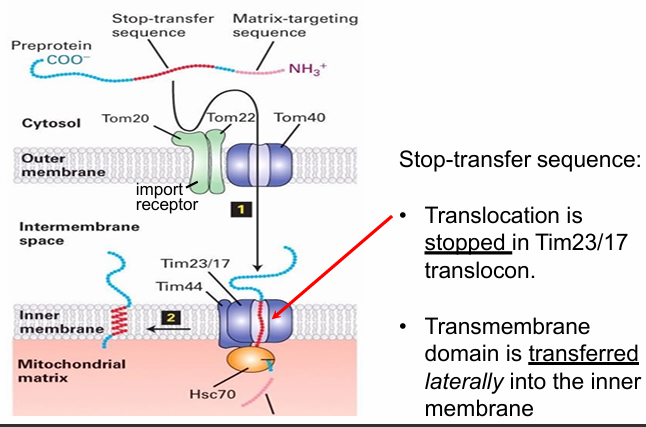
Protein Transport to Inner Mitochondrial Membrane
Initially same as transport to the matrix
N-terminal motif recognized by import receptor
N-terminus translocated through the Tom and Tim translocons
The N-terminus of mitochondrial protein does go to the mitochondrial matrix
However, the Tim also recognizes the stop-transfer sequence
Stop-transfer sequence forms an hydrophobic alpha helix that does 2 things
1. Stop the translocon so protein is no longer pulled through
2. Directs the transfer of the protein out of the translocon and into the inner membrane
The translocon open laterally (sideways)
Creates an opening exposing the stop-transfer sequence to the hydrophobic env of inner membrane
The proteins then imbeds into the inner membrane
Is the stop-transfer sequence necessary for inner membrane transport?
Yes
The protein would still get into the matrix due to the matrix-targeting sequence
The protein won’t be able to embed itself into the inner membrane though
So it’s necessary for transport to the inner membrane but not for transport to matrix
Is the stop-transfer sequence sufficient for inner membrane transport?
Tagging a cytosolic protein (Ex. GFP) with the stop-transfer sequence
If you add JUST the stop-transfer sequence, the protein stays in cytosol
So it’s not sufficient for transport to inner membrane
It’s necessary though
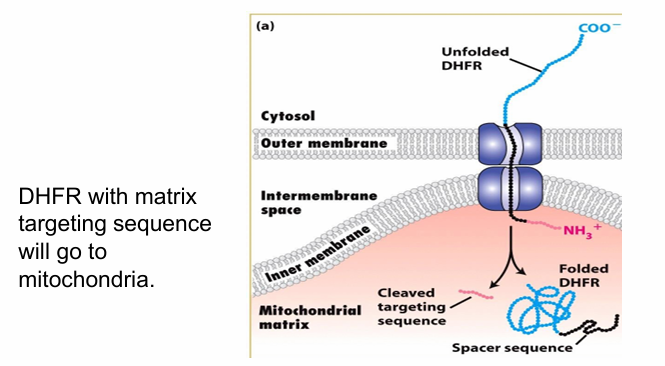
Is unfolding necessary for protein transport to mitochondria? (DHFR)
Target cytosolic protein for transport (DHFR shown in blue)
Tag it with matrix-targeting motif (red) and add a spacer sequence (black)
Length of 2 translocons
Presence of Hsc70 keeps DHFR unfolded allowing it to be transported into the mitochondrial matrix
So any unfolded protein is getting through, so long as it has the matrix-targeting motif atleast which is sufficient for transport
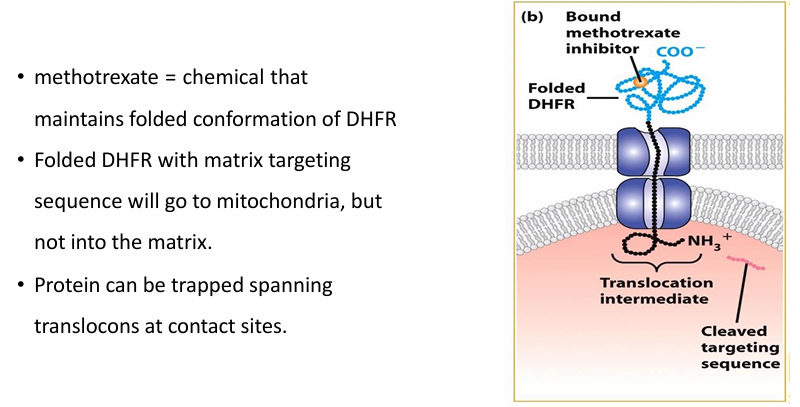
Is unfolding necessary for protein transport to mitochondria? (DHFR and Methotrexate)
Methotrexate maintains folded confromation of DHFR despite Hsc7-
Matrix-targetinig motif is still sucessful in getting the protein to mitochondria
Some of the protein is pulled through translocoon
The spacer sequence gets through
the matrix-targeting sequence gets cleaved
Since DHFR is folded, it cannot get through
Protein unfolding is therefore necessary for transport of proteins into mitochondria
Defects in Mitochondrial Transport
Causes:
Mutations in target signals
Mutations disrupting import machinery
Deficiencies in the chaperone Hsc70
Can effect any system in body
Often associated with neurodegeneration though
Post-Translational Targeting to other Organelles
Chloroplast:
Uses N-terminal targeting motif
Nucleus
Uses C-terminal nuclear localization sequence