Chem Ch 6
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Microwave Radiation
Molecular rotational levels
Infrared radiation
molecular vibrational levels
Ultraviolet-visible radiation
electronic energy levels
What are the problems with wave theory
Emission of light from hot objects
The photoelectric effect
Emission spectra
What is the Photoelectric Effect
Phenomenon where light shining on a metal surface ejects electrons from the metal
Evidence for the particle nature of light
threshold frequency must be reached
Below this, no electrons are ejected
Above this, the # of electrons ejected depends on the intensity of the light
Spectra tube
Emits light unique to the element in it
Only a few wavelengths are seen
Black regions are wavelengths that are absent
3 Postulates for Bohr’s theory of the atom
Electrons move in orbits that have defined energies
An electron in an orbit has a specific energy
Energy is only emitted or absorbed by an electron as it changes from one allowed energy state to another
Limitation of the Bohr Model
Only explain the line spectrum of hydrogen
Electrons are not completely described as small particles
Doesn’t account for the wave properties of electrons
deBroglie equation
wavelength = Planck constant/mass*velocity
suggested that an electron moving about the nucleus of an atom behaves like a wave and therefore has a wavelength
Heisenberg’s Uncertainty Principle
We can’t determine the exact position, direction of motion, and momentum of an electron simultaneously
Erwin Schrodinger
Proposed an equation that contains both wave and particle terms
Solving the equation leads to wave functions (shape of the electron orbital)
Orbitals
regions of highly probable electron locations
What 3 quantum numbers does Shrodinger’s equation require?
Principal Quantum Number: n
Azimuthal Quantum Number: l
l = n - 1
use letters s, p, d, and f for l
Magnetic Quantum Number: ml
Dependent on l, values between -l to +l
s-Orbitals
Spherical
As n increases, the s-orbitals get larger
p-Orbitals
three p-orbitals: px, py, and pz
Correspond to allowed values of ml of -1, -, and +1
Orbitals are dumbbell shaped
As n increases, p-orbitals get larger
Orbitals and their energies
Orbitals of the same energy are said to be degenerate
For n ≥ 2, the s- and p-orbitals are no longer degenerate because the electrons interact with each other
Therefore, the Aufbau diagram looks slightly different for many-electron systems
Pauli’s Exclusion Principle
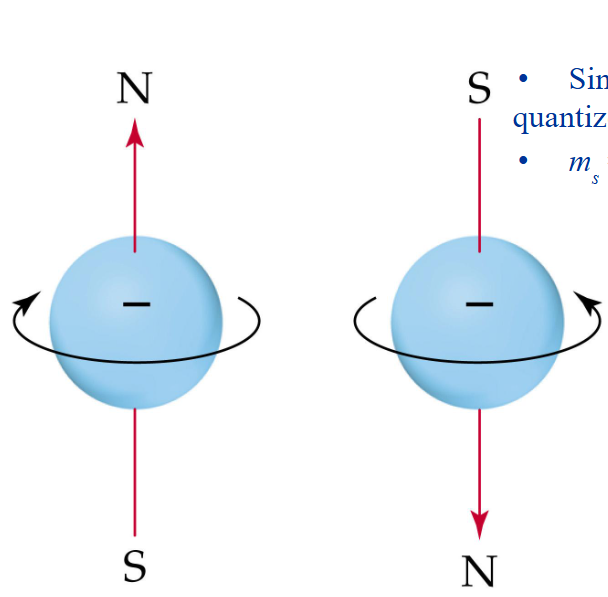
Since electron spin is also quantized, we define
ms = spin quantum # = ± ½
no two electrons can have the same set of 4 quantum numbers
Therefore, two electrons in the same orbital must have opposite spins
Electron capacity of sublevel = 4l + 2
Electron capacity of energy level = 2n2
Hund’s Rule
for degenerate orbitals, electrons fill each orbital before any orbitals get a second electron
degenerate Orbitals
Orbitals of the same energy
paramagnetic atom
one or more unpaired electrons
paramagnets do not retain magnetization in the absence of a magnetic field, this is because thermal energy randomizes electron spin orientations
diamagnetic atom
all electrons are paired
repel magnetic fields
unpaired electrons of paramagnetic atoms realign in response to external magnetic fields and are therefore attracted
Electron config notation
energy level, subshell, # of electrons per orbital
Orbital notation
each ml value is represented by a line, electrons are also shown
Noble gas configuration/ “condensed config”
[Preceding noble gas] electron config notation for outer shell electrons
valence electrons