Transdermal drug delivery 1
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
What does “topical application for systemic effect” mean?
Applying a drug to the skin to achieve effects throughout the body, not just at the site of application.
How does topical systemic delivery differ from local topical delivery?
Topical systemic delivery targets the whole body, while local topical delivery acts only where applied.
What is the typical absorption pattern of an orally administered drug
Absorption is usually rapid, leading to a quick rise in plasma concentration.
In oral delivery, how do the kinetics of absorption compare to elimination?
Absorption is typically faster than elimination.
What happens to plasma concentration (Cp) after oral administration?
: Cp increases rapidly to a maximum (Cmax), then decreases as the drug is eliminated.
What determines the rate at which plasma concentration falls after reaching Cmax? for oral drug delivery
The drug’s elimination kinetics and half-life.
Why can’t high Cp levels be maintained long after oral dosing?
Because the drug is eliminated faster than it can be maintained, requiring re-dosing.
What is a drawback of oral drug delivery in maintaining steady plasma levels?
It causes peaks and troughs in Cp due to rapid absorption and elimination.
How does topical (transdermal) systemic delivery improve plasma concentration control?
It allows slow, controlled absorption through the skin, keeping Cp more constant over time.
What are the benefits of topical systemic delivery compared to oral delivery?
More stable plasma levels, fewer side effects, improved convenience, and less frequent dosing.
What is the main goal of topical application for systemic effect?
To deliver a drug through the skin into the bloodstream for whole-body (systemic) action.
How does the absorption rate of topical delivery compare to oral delivery?
Absorption through the skin is much slower than absorption from the gut.
In topical systemic delivery, which process is slower: absorption or elimination?
Absorption is slower than elimination.
What is the term used when absorption is slower than elimination?
: “Flip-flop kinetics.”
What are “flip-flop kinetics”?
A situation where the rate of absorption, not elimination, determines how long the drug stays in the body.
Describe the plasma concentration (Cp) profile after topical administration.
Cp increases slowly to a lower maximum and then declines gradually over time.
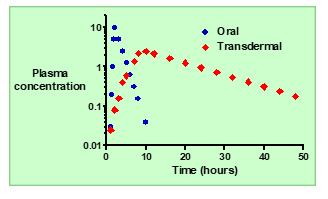
How does the apparent half-life (t½) of a topically administered drug compare to oral dosing?
It appears longer because absorption continues while elimination occurs.
Why is a high plasma concentration (Cp) not achieved with topical systemic delivery?
Because absorption is slow and spread out over time, preventing sharp peaks.
What are the benefits of the slow absorption seen in topical systemic delivery?
Sustained plasma levels, improved control of drug concentration, and less frequent dosing.
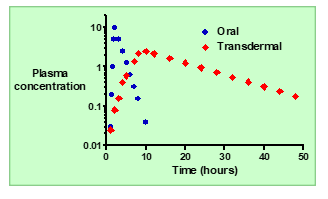
Summarize the main advantage of topical systemic delivery over oral dosing.
It provides steadier, longer-lasting drug levels with fewer side effects and reduced dosing frequency.
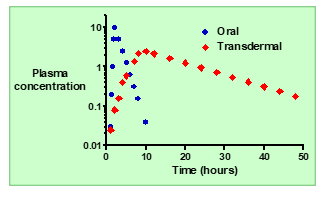
Why are potent drugs good candidates for topical systemic delivery
Because they require only small doses to achieve therapeutic effects, making sustained absorption through the skin feasible.
What is the main pharmacokinetic advantage of topical systemic delivery?
It maintains sustained plasma concentrations over time.
How does topical systemic delivery achieve sustained drug levels?
Through slow, continuous absorption of the drug across the skin into the bloodstream.
What does “control of delivery” mean in the context of topical systemic drugs?
The ability to regulate the rate and duration of drug release by adjusting patch design or formulation.
How does topical systemic delivery affect dosing frequency?
It reduces dosing frequency because the drug is released gradually over a long period.
Why might topical systemic delivery cause fewer side effects?
Because it avoids high plasma peaks that can exceed toxic levels, keeping concentrations within the therapeutic window.
What is MEC (Minimum Effective Concentration)?
The lowest plasma concentration needed to produce a therapeutic effect.
What is MTC (Minimum Toxic Concentration)?
he plasma concentration above which adverse or toxic effects occur.
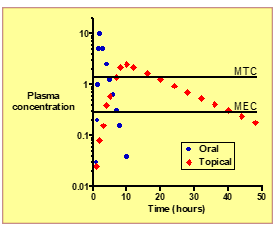
Define the Therapeutic Index (TI).
The ratio of MTC / MEC, representing the drug’s safety margin.
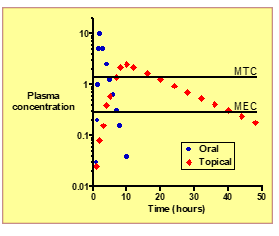
How does topical systemic delivery affect the therapeutic window compared to oral dosing?
: It keeps plasma levels within the window (between MEC and MTC) more consistently, improving safety and efficacy.
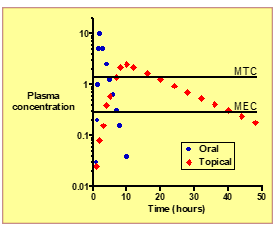
What is the overall clinical advantage of topical systemic delivery?
Sustained, controlled drug levels with fewer side effects and less frequent dosing.
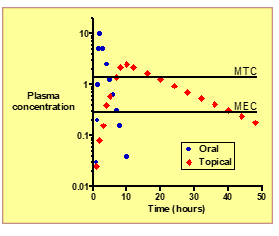
What is the primary use of Glyceryl Trinitrate (GTN)?
To treat angina pectoris by causing vasodilation and improving blood flow to the heart.
How is GTN typically administered for acute angina relief?
Through a sublingual tablet, placed under the tongue for rapid absorption.
What is the onset of action for GTN sublingual tablets?
Very fast — within 1–3 minutes after administration.
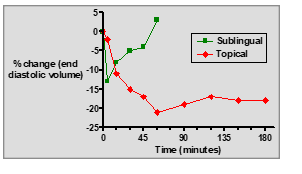
What pharmacokinetic advantage does the sublingual route of GTN offer?
It partially avoids the first-pass effect, allowing more drug to enter systemic circulation.
What is the half-life (t½) of GTN when taken sublingually?
2–3 minutes — GTN is rapidly metabolized.
How long does the effect of a 0.4 mg GTN sublingual tablet last?
The effect is short-lived, typically lasting about 20–30 minutes
: How is GTN ointment administered for angina prevention?
: It is applied to the skin (typically on the chest) as a 2% w/w ointment.
What is the typical dose range for GTN ointment?
: 1–5 cm of ointment (equivalent to 7.5–30 mg) applied to the skin.
How does the onset of action for GTN ointment compare to sublingual tablets?
Slower onset (15–60 minutes) compared to the fast onset of sublingual administration.
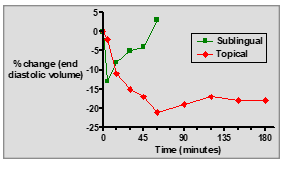
What is the advantage of using GTN ointment over sublingual tablets?
: It provides a sustained effect over a longer period with controlled release into the bloodstream.
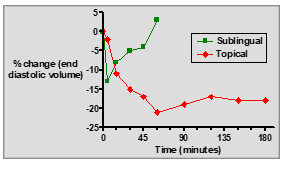
What is a major disadvantage of GTN ointment compared to sublingual tablets?
It requires a larger dose and has a slower onset of action.
How does GTN ointment provide controlled drug delivery?
the drug is absorbed slowly through the skin, allowing for steady, sustained plasma levels.
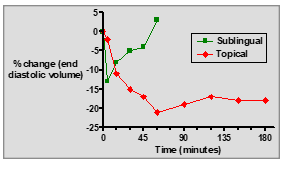
What are the advantages of using topical GTN (ointment) for angina prevention?
Sustained effect, controlled absorption, and reduced dosing frequency.
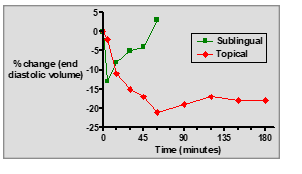
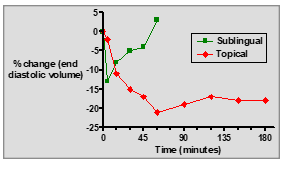
What are the key differences between sublingual GTN tablets and topical GTN ointment?
Sublingual tablets: Fast onset, short duration, rapid relief for acute angina.
Topical ointment: Slow onset, longer duration, sustained prophylactic effect.
What is the first-pass effect in pharmacology?
: The initial metabolism of a drug in the liver after it’s absorbed from the gastrointestinal tract before it reaches the systemic circulation.
How does the first-pass effect impact a drug's bioavailability?
It reduces bioavailability because a portion of the drug is metabolized in the liver before entering the bloodstream.
What organ is primarily responsible for the first-pass effect?
The liver, which metabolizes drugs absorbed from the gastrointestinal tract.
How does the liver metabolize drugs during the first-pass effect?
The liver enzymes, mainly the cytochrome P450 enzymes, break down the drug, potentially inactivating it or converting it into metabolites.
Why is the first-pass effect significant in determining drug dosing?
Because it reduces the amount of the drug that enters circulation, meaning higher doses may be needed if taken orally.
: How can the first-pass effect be avoided or minimized?
: By using routes of administration that bypass the liver, such as sublingual, intravenous, or transdermal routes.
Why is sublingual administration used for GTN
ecause it bypasses the liver, avoiding the first-pass effect and allowing the drug to enter systemic circulation more efficiently.
Why is transdermal delivery generally not feasible for biotech drugs?
Biotech drugs (like proteins and monoclonal antibodies) are often too large, hydrophilic, and unable to pass through the skin.
What are the main advantages of transdermal drug delivery?
Non-invasive route.
Sustained release for steady plasma drug levels.
Avoidance of first-pass metabolism (leading to higher bioavailability)
How do microneedles improve transdermal drug delivery?
Microneedles create tiny, painless microscopic channels in the skin, allowing larger molecules (like biotech drugs) to pass through and enter the bloodstream.
offers a non-invasive, painless alternative to injections.
What does avoiding pre-systemic metabolism in transdermal drug delivery lead to?
It results in a higher bioavailability of the drug, allowing for a lower daily dose compared to oral administration.
How does transdermal delivery help with lower daily doses?
By bypassing first-pass metabolism, the drug is more effective, so lower doses can achieve the same therapeutic effect.
How does transdermal drug delivery help maintain drug levels within the therapeutic window for a prolonged period?
Transdermal systems provide controlled release, keeping the drug at consistent levels within the therapeutic window, ensuring both efficacy and safe
How does transdermal drug delivery reduce inter- and intra-patient variability?
It offers predictable, controlled delivery, reducing fluctuations in how the drug is absorbed and metabolized by different patients or the same patient over time.
Why does transdermal drug delivery improve patient compliance?
: It requires fewer doses, is easy to apply, and improves convenience, making it easier for patients to follow the prescribed regimen.
What is the benefit of drug input being terminated simply by patch removal?
If therapy needs to be stopped, it’s as simple as removing the patch, which immediately halts the drug’s delivery.
Why is transdermal delivery limited to potent drugs?
Transdermal systems are typically effective only for drugs with small daily doses (≤ 50 mg) because higher doses may not be absorbed effectively through the skin.
What type of drug molecules are suitable for transdermal delivery?
The drug should be small in size and lipophilic (fat-soluble), but it must also be soluble in both oil and water for effective absorption.
: What are the pharmacokinetic limitations of transdermal drug delivery?
transdermal delivery works best with drugs that have a suitable half-life, are not rapidly metabolized, and can be dosed in a sustained release regimen.
What is the typical size limit for transdermal patches?
: The patch size is generally limited to ≤ 100 cm², meaning only a limited amount of drug can be delivered at once.
What skin reactions must be avoided in transdermal delivery?
The drug and patch must not cause local irritation or sensitization (e.g., allergic reactions) at the application site.
What are the efficiency and expense limitations of transdermal drug delivery?
Transdermal systems can be inefficient for drugs that require high doses, and they may be more expensive than oral formulations, making their use less justifiable unless significant benefits (e.g., controlled release) are provided.
How does transdermal drug delivery overcome the lipid bilayer of skin cells?
Lipophilic drugs can easily diffuse through the lipid bilayer of skin cells, while hydrophilic drugs need penetration enhancers (e.g., ethanol) to temporarily disrupt the barrier, allowing the drug to pass through.
What is the main feature of adhesive systems in transdermal drug delivery?
The drug is incorporated into the adhesive layer, which directly sticks to the skin, allowing the drug to diffuse continuously through the skin into the bloodstream.
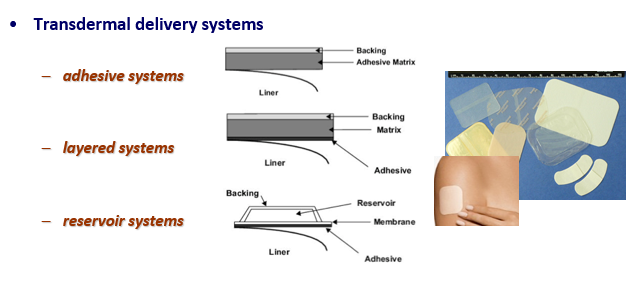
What are the key features of layered systems for transdermal drug delivery?
Layered systems have a drug layer and rate-controlling layers that regulate the release of the drug, providing steady, controlled release over time.
How do reservoir systems work in transdermal delivery?
Reservoir systems contain a drug reservoir, and the drug is released through a rate-controlling membrane to ensure steady, consistent release over a long period.
What is the role of polymers in transdermal delivery systems?
Polymers form the drug matrix, help control drug release, and ensure the stability and sustained delivery of the drug.
What are pressure-sensitive adhesives (PSAs) used for in transdermal delivery systems?
PSAs ensure the patch adheres to the skin, maintain contact, and control the rate of drug release.
What materials are commonly used for release liners in transdermal patches?
ilicones, polyesters, and polycarbonates are used for release liners, which protect the patch until it is ready to be applied.
What is the purpose of backings and laminates in transdermal delivery systems?
Backings protect the drug and adhesive from external factors and provide structural integrity, using materials like EVA, polypropylene, and polyester.
What are the most common vehicles used in transdermal patches?
Mineral oil, fatty acid esters, PEG, glycerin, ethanol, and silicone oil help solvate the drug and enhance skin penetration
What are some examples of other excipients in transdermal systems?
Lactose (bulking agent) and cross-linkers (for polymer binding) are used to stabilize and control drug release.
example 1: gtn
What are the key characteristics of GTN (Glyceryl Trinitrate) for transdermal delivery?
GTN has a short t½, = short duration of action, requiring continuous delivery.
high clearance (Cl),= quickly eliminated from the body.
low oral bioavailability (F), = Oral bioavailability is poor, making it unsuitable for oral administration but ideal for transdermal delivery.
log P ~ 2, = The drug is moderately lipophilic, allowing it to pass through the skin.
MW ~ 300, =making it small enough for skin absorption.
and is liquid at room temperature, which can facilitate easier formulation in patches.
How much GTN can a 50 cm² transdermal patch deliver in 24 hours?
A 50 cm² patch can deliver 25 - 50 mg of GTN in 24 hours.
What is the potential issue with GTN transdermal delivery related to tolerance?
Tolerance can develop with continuous use, requiring a 12-16 hour washout period after wearing the patch.
Continuous Exposure means body becomes less responsive to drug.
Reduced Effect: After wearing the patch for a while, the body becomes less sensitive to the effects of GTN (such as vasodilation and relief from angina).
Washout Period: To prevent or reduce tolerance, users are typically instructed to remove the patch for a "washout" period of 12-16 hours between patches. This allows the body to "reset" and restore its response to the drug.