[BCHEM] BLOOD GENERALITIES & RBC METABOLISM
1/200
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
201 Terms
transport, regulate, and protection
What are the major functions of the blood
red blood cells, platelets, white blood cells, and plasma
What are the four main components of the blood
hemoglobin, heme protein
This is the principal determinant of blood color, attributed to the presence of oxygen bound to which protein
planar structure, bright red
oxygen binds with the heme iron to create (this particular structure of hemoglobin) and appears as what (color)
non-planar stucture
The blood darkens when deoxygenated and creates which kind of stucture
Infants and neonates
Volume of Blood in
1) Infants and neonates 2) Adults
Males
Volume of Blood in
1) Males 2) Females
Serum
Which liquid portion when centrifuged does not contain anticoagulants?
Plasma
Which liquid portion when centrifuged contain anticoagulants, which prevents clotting of blood?
as a fibrin clot
The formed elements in serum are seen as which structure on top of the centrifuged whole blood?
they are easily separated and still suspended (due to anticoagulants added)
What is the characteristic of the formed elements in the plasma that differs from serum?
red blood cells
What are the heaviest/most dense elements in the blood that make up the majority of the formed elements?
Buffy coat
This is a thin white, middle layer of a centrifuged whole blood
WBC, Platelets
Buffy coat contains which elements?
plasma (55%) and formed elements (45%)
In the circulation, whole blood is composed of
fibrinogen
Serum is only formed during blood extraction when clotting factors, specifically ( ) are removed from plasma
Water (majority), Solutes (Organic, and Inorganic compounds)
Composition of the plasma of the whole blood in the circulation?
Hematopoiesis
This refers to the synthesis of blood cells
hematopoietic stem cells
All formed elements from blood have a common precursor derived from
bone marrow
These cells migrate from sites of hematopoiesis to reside in which structure
Cytokines
These are a subtype of growth factors and are cell-signaling molecules that mediate and regulate immunity, inflammation, and hematopoiesis
JAK-STAT Pathway
Most of the cytokines are recognized by the receptors which activate which pathway?
phosphorylated
Janus Kinases (family of tyrosine kinases) are usually active when in this state
dimerization and activation of JAKs
Step 1 in JAK STAT Pathway involves cytokine binding to receptors initiating which processes that favors phosphorylation of JAK tyrosine residues
binding of additional signal transduction molecules to the STAT
Step 2 in JAK STAT Pathway involves JAK dimerization and phosphorylation that promotes
gene transcription
Step 3 of JAK STAT Pathway: Binding and phosphorylation of STAT promotes its dimerization and its translocation to the nucleus, which then activates?
F; promote proliferation
T/F: Activation of gene transcription would then inhibit proliferation of specific cell lineages from the hematopoietic stem cells
Silencer of Cytokine Signaling (SOCS) proteins (4&5)
What are the negative regulators of the JAK-STAT pathway that may either bind to the phosphorylated receptors or bind to the JAKs itself and inhibit them
common myeloid progenitor cells
Hematopoietic stem cell proliferation & differentiation produce?
Stem cell factor (SCF) and Granulocyte-monocyte colony-stimulating factor (GM-CSF)
Hematopoietic stem cell proliferation & differentiation into common myeloid progenitor cells are stimulated by
RBCs - erythropoietin (EPO)
Platelets - thrombopoietin
Granulocytes and monocytes - Stem cell growth factor (GM-CSF + IL-5,6)
Myeloid progenitor cells then differentiate into:
RBCs by
Platelets by
Granulocytes and monocytes by
Common Lymphoid Progenitor cells
HSCs also form this lineage that eventually formsi B and T lymphocytes
Hematocrit Level
This refers to the volume of erythrocytes compared to the total blood volume (proportion of blood by volume consisting of RBC)
T
T/F: Hct level is directly proportional to the RBC count
Hemoglobin
This represents the protein component within each RBC and delivers oxygen to the organs and tissues; carries CO2 to lungs
Anemia
This occurs when Hgb concentration is below normal values
Mean Corpuscular Volume (MCV)
Red cell size is measured by?
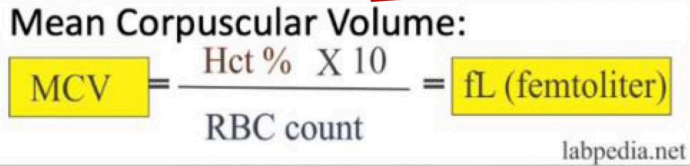
NV: 80-100 fL
Normal value and Formula of MCV
Mean Hemoglobin Concentration (MCHC)
Hemoglobin concentration is measured by

NV: 32-36 g/dL
Normal value and Formula of MCHC
heme iron from animal foods
Which is better absorbed, non-heme iron from plant-based iron or heme iron from animal foods?
Ferroportin
These are proteins within the intestinal cells that will facilitate the reabsorption of iron
Transferrin
This protein carries iron around the circulation because iron in its ferrous form is toxic
Ferritin and Hemosiderin
These proteins bind with storage iron (storage form) will give it back to transferrin to be delivered to the bone marrow if needed for hemoglobin synthesis
Hephaestin
Copper-containing protein in the intestinal cells that also assists in the reabsorption of iron
ferroxidase activity
Why does ferroportin require hephaestin
oxidation of ferrous (Fe2+) to ferric (Fe3+)
Ferroxidase activity of hephaestin with ferroportin will facilitate which process for proper transport of iron for heme synthesis, allowing it to bind to transferrin
Ceruloplasmin
Major Cu++ carrier protein in blood that facilitates transport of Cu++ and also has ferroxidase activity
Copper Deficiency
This condition results into decrease of iron transport → iron accumulation in tissues
Hephaestin and Ceruloplasmin
Recall: What are the two proteins that have ferroxidase activity?
Coenzyme in the synthesis of heme, particularly in the enzyme aminolevulinic acid (ALA) dehydratase to produce porphobilinogen (PBG)
Folic Acid (Vit B9), Cobalamin (Vit B12)
These are essential for DNA synthesis and critical in the synthesis of new cells, including the RBCs
proerythroblast
EPO will stimulate synthesis first ( ) which also marks the start of hemoglobin production
erythroid committed precursors
In erythropoiesis, multipotent (common) myeloid progenitor cells commit to differentiate into
Reticulocytes and Pyrenocytes
Eventually the erythroblast will lose its nucleus and some organelles forming two daughter cells
T
T/F: Reticulocytes retain their capacity to synthesize polypeptides (eg. globin) because they have some ribosomes and mRNAs
F; Devoid of organelles and are unable to reproduce by itself and thus requires erythropoietin stimulation for synthesis of new RBCs
T/F: Mature erythrocytes contain organelles and therefore can reproduce by itself
Hemoglobin
What comprises 95% of the intracellular protein of red blood cells
Unique RBC cytoskeletal network enables it to fold over and squeeze through
The RBC membrane is strongly flexible and has reversible deformation due to which characteristic?
intracellular hemoglobin concentration
Viscosity of the cytoplasm of RBC is dependent on?
Phospholipids, Sphingolipids, Cholesterol, Integral membrane proteins
Lipid bilayer of RBC includes
Band 3, Glycophorins
What are the integral membrane proteins found in the lipid bilayer of RBC
tropomyosin-tropomodulin junctional complexes
Cytoskeleton of RBC, which consist of spectrin tetramers, is connected to actin via
Band 3
Cytoskeleton of RBC is tethered to the lipid bilayer through this protein at the spectrin-ankyrin binding sites
Adducin
This forms bridge between RBC bilayer and cytoskeleton
Accumulate Oxidative Damage
This reason of RBC Aging is caused by cycle of gas exchange and exposure to extrinsic chemicals which accumulates in the proteins within RBC
Cytoskeletal Damage
This reason of RBC Aging is due to osmotic swelling and shrinkage as well as gradual loss of surface area → decreases deformability and repetitive deformation
Absence of organelles
Reason why RBC is unable to repair cytoskeletal proteins and unable to synthesize new hemoglobin
Spleen and liver; macrophages
Aged RBCs are removed in these organs by which cells?
Hemoglobin components are further broken down, recycled, or metabolized into
Globin
Protein portion of hemoglobin that is broken down into amino acids and is sent back to bone marrow for the production of new RBCs
T
T/F: Recycled Iron is stored in the liver or spleen as ferritin and hemosiderin and is carried by the bloodstream by transferrin to the bone marrow
Biliverdin
Heme is degraded into this green pigment that will eventually be converted to bilirubin
Bilirubin
Degraded product of heme that appears as a yellow pigment and the discoloration often associated in cases of jaundice
albumin
Bilirubin binds to this protein to travel via bloodstream to the liver for bile production
urobilinogen and stercobilin
In the large intestines, bilirubin is broken apart from bile by bacteria and converts it to
urine
In the kidneys, bilirubin and other related byproducts (urobilin) are eliminated through
Biliverdin
This degraded component of heme is responsible for the dramatic colors associated with bruising
T
T/F: Body assumes a yellow-tinged color if you block bilirubin before or after the liver
no mitochondria
Reason why mature RBCs have no TCA cycle, ETC, β-oxidation pathway, No oxidative phosphorylation
No nucleic acid and protein synthesis
What are the consequences of RBC having no nucleus and ribosomes?
Maintenance of electrolyte gradient, synthesis of glutathione, maintenance of ferrous (Fe2+) state, and maintain the biconcave shape of RBCs
Mature RBCs require energy to maintain several functions
GLUT-1 transporter
Glucose is taken up by the RBC via
high affinity, NOT affected by insulin
GLUT-1 transporter has high/low affinity glucose and is/not affected by insulin
Glycolysis (EMP) - anaerobic; net gain of 2 ATP
Major utilization of the glucose supply of RBC and what is its net gain of ATP
Hexokinase (Step 1), Phosphofructokinase (Step 3)
Two ATP-consuming steps of the Preparatory Phase of Glycolysis
Phosphoglycerate kinase and Pyruvate kinase
Two ATP-generating steps of the Payoff Phase of Glycolysis
Lactate Dehydrogenase, G3PD step
This reduction of pyruvate to lactate through this enzyme subsequently regenerates NAD, which can be reutilized in this step and in turn generates NADH
Triosephosphate Isomerase
Important enzyme in EMP that exhibits the highest glycolytic enzyme activity to isomerize DHAP to G3PO4 → enter the payoff phase
G3PO4 dehydrogenase
Transforms G3P to 1,3-BPG with NAD as a cofactor (which comes from the conversion of pyruvate to lactate via LDH)
1,3-BPG (intermediate to 2,3-BPG)
Products of EMP that serves as a regulator (regulatory intermediate) of oxygen affinity to hemoglobin
NADH
Products of EMP that converts iron to its functional ferrous state
Rapoport-Luebering Shunt
Unique branch of the glycolytic pathways seen in mature RBCs
Rapoport-Luebering Shunt generates and dephosphorylates 2,3-bisphosphoglycerate (2,3-BPG) via this enzyme
Isomerize 1,3-BPG to 2,3-BPG
Mutase activity of BPGM by bisphosphoglycerate mutase
Hydrolysis of 2,3-BPG to 3-PG (→ reenter the main glycolytic pathway)
Phosphatase activity of BPGM by bisphosphoglycerate phosphatase
2,3-BPG
This facilitates the supply of oxygen to the tissues by binding to hemoglobin
R-state
When hemoglobin is deoxygenated, the equilibrium is driven to this state in which majority of O2 will attach to Hgb (oxygen loading)
oxygen unloading (decreases O2 affinity to Hgb)
In the presence of 2,3-BPG, it binds to the center pocket of the 2 beta-globin chains of Hb → stabilizes hemoglobin in the T-state, which favors?
GSH Reductase
Enzyme to reduce GSSG into its sulfhydryl form (GSH) through disulfide cleavage