ABE Notation: Understanding Shape and Bond Angles in Geometry
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
AB2
Linear; 180°; sp
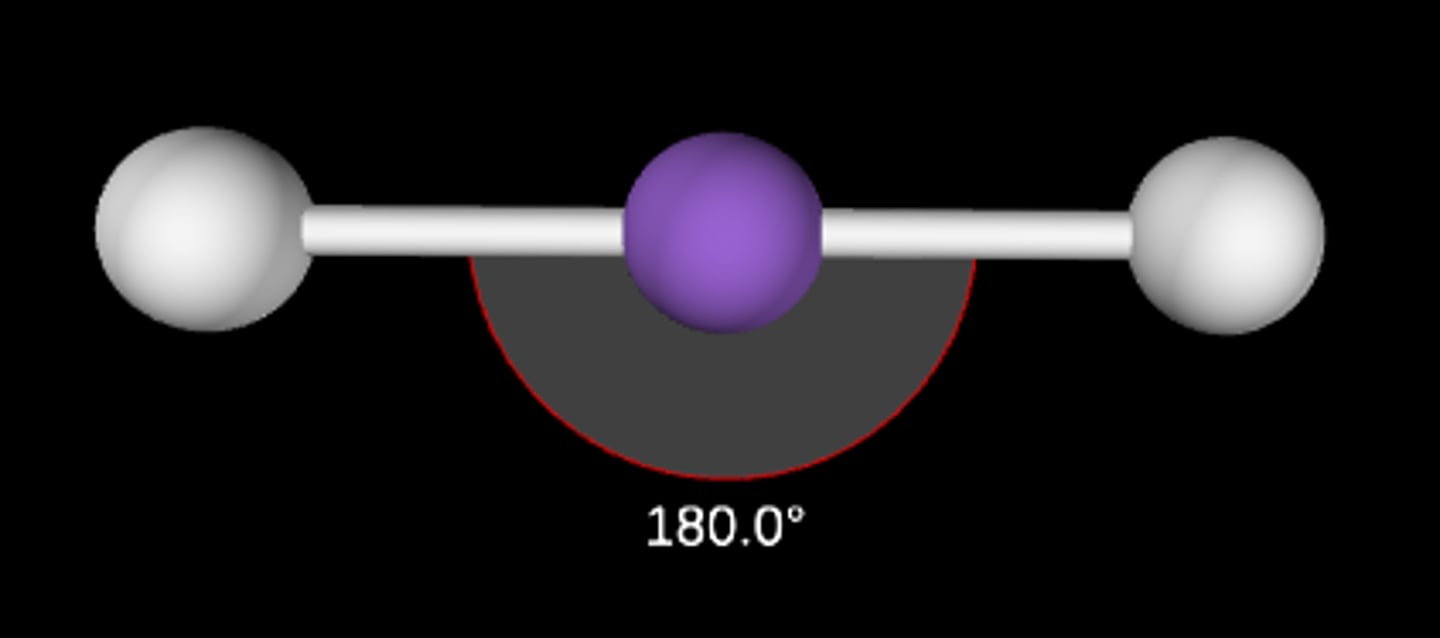
ABE
Linear; 180°; sp
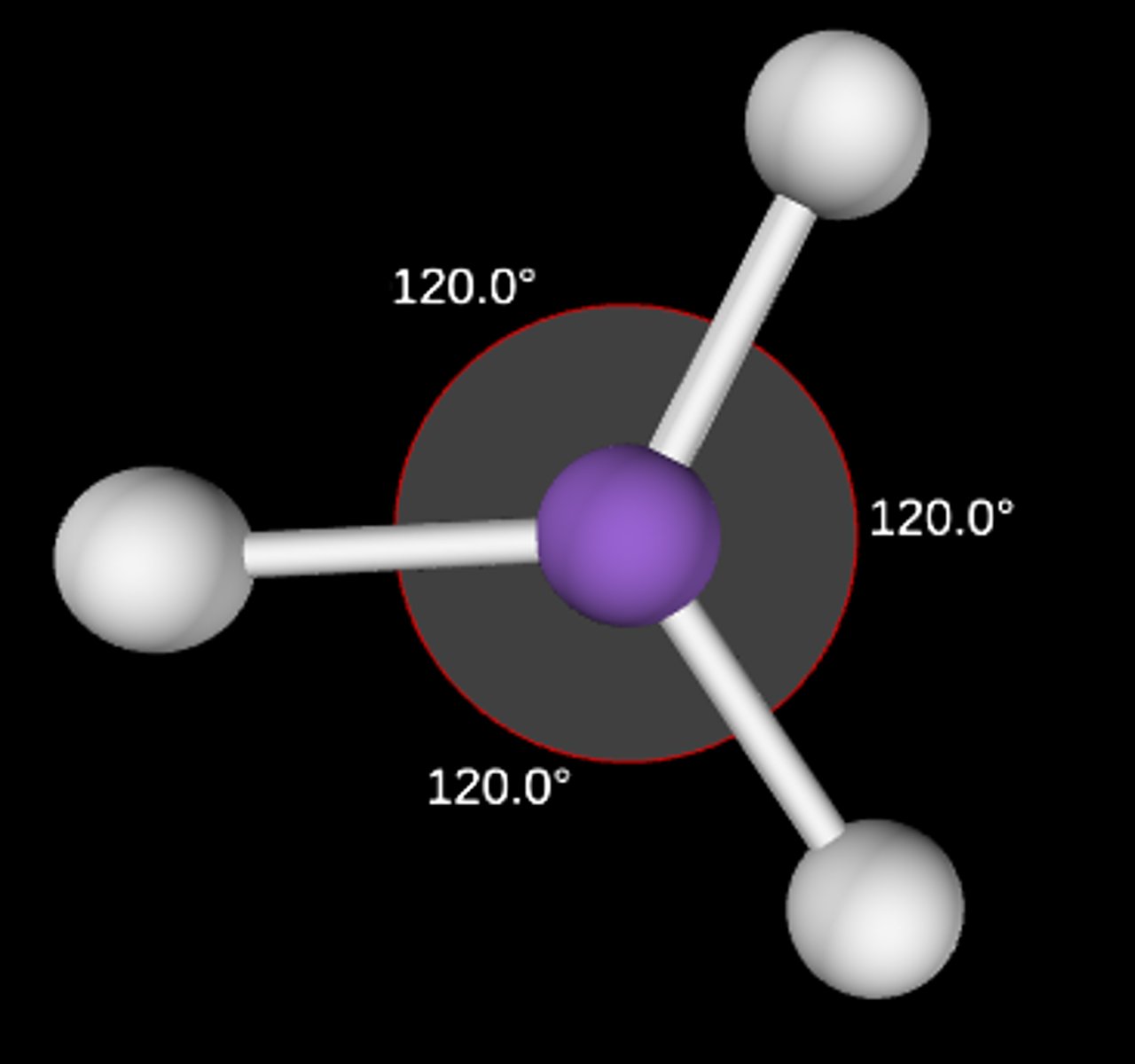
AB3
Trigonal planar; 120°; sp2
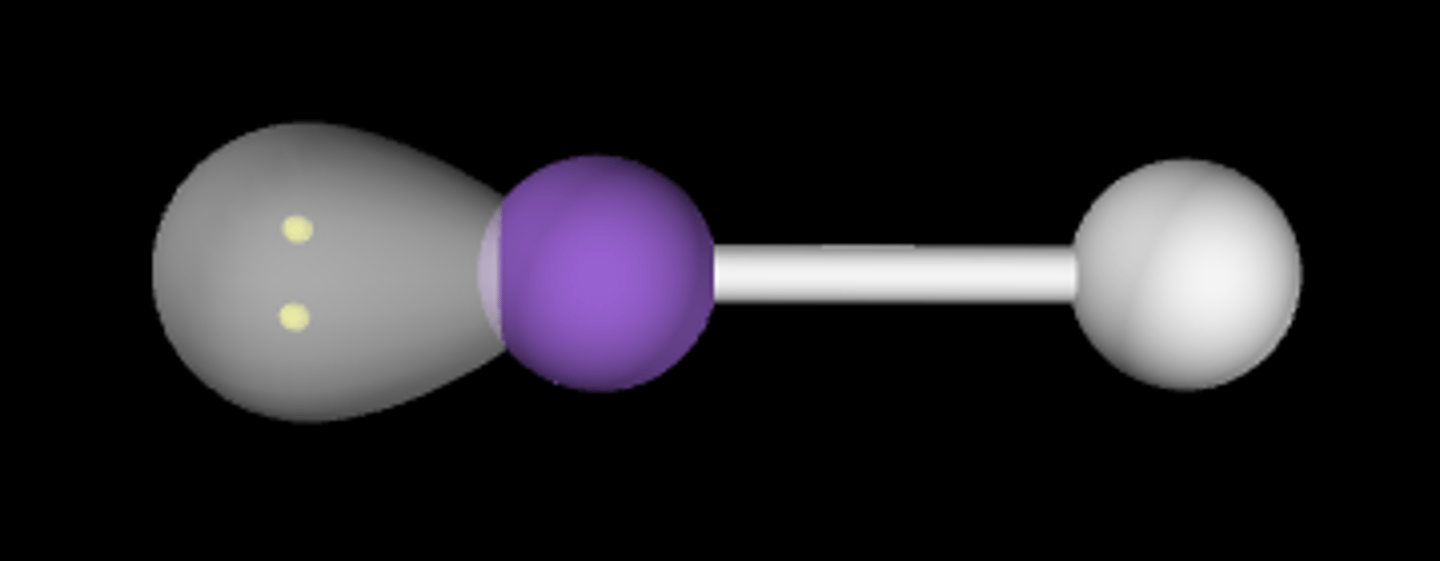
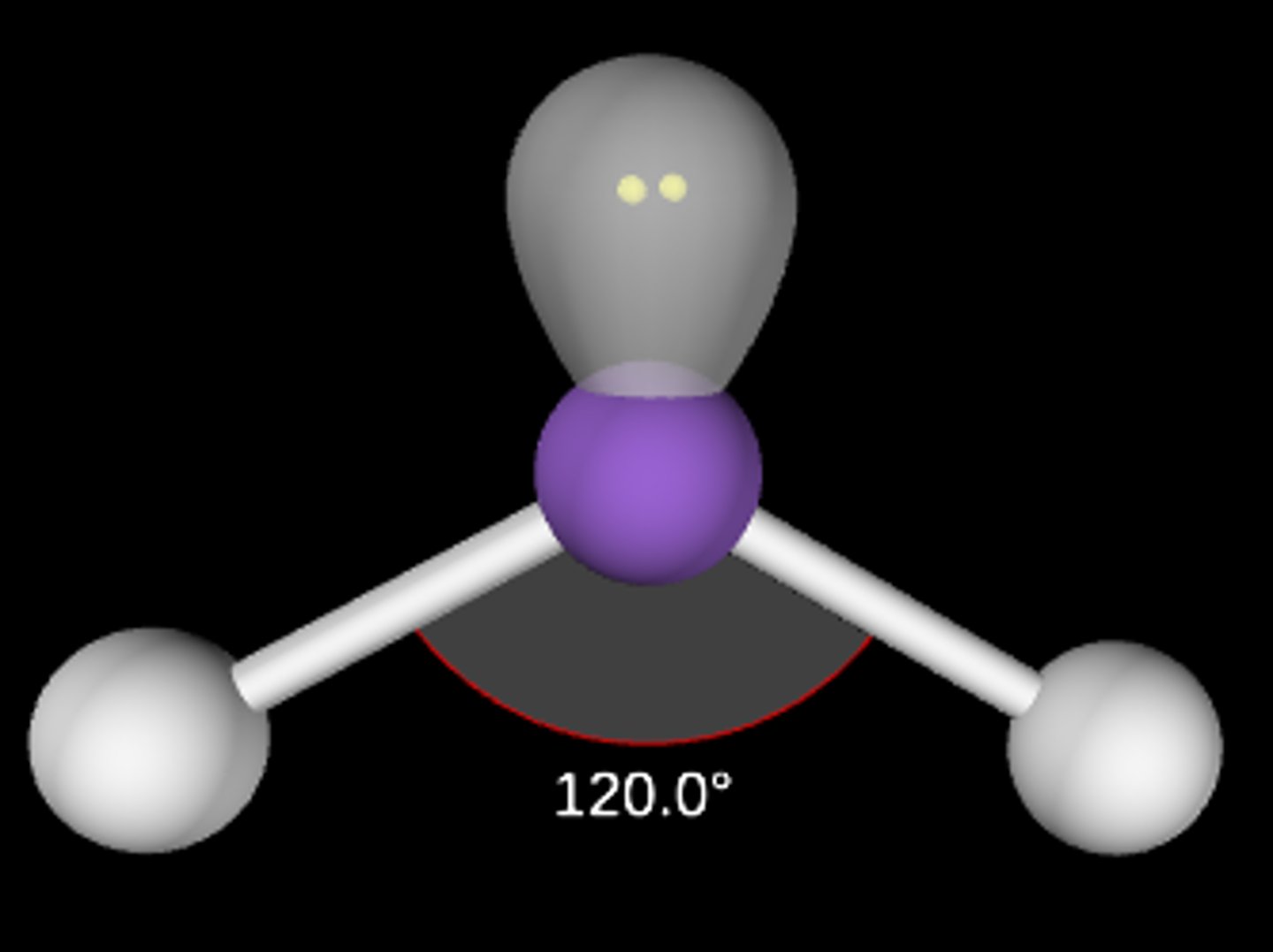
AB2E
Bent/angular; < 120°; sp2
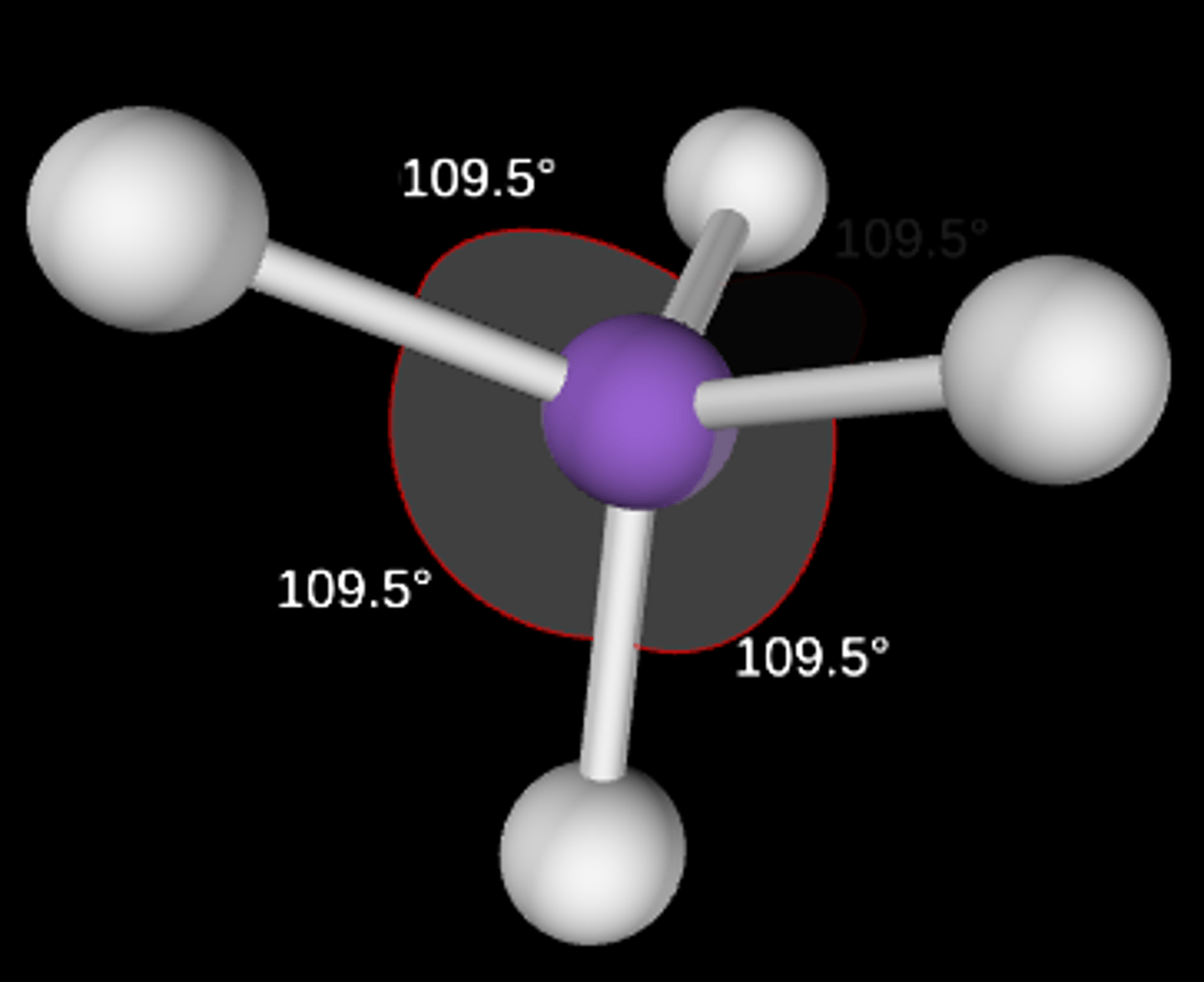
AB4
Tetrahedral; 109.5°; sp3
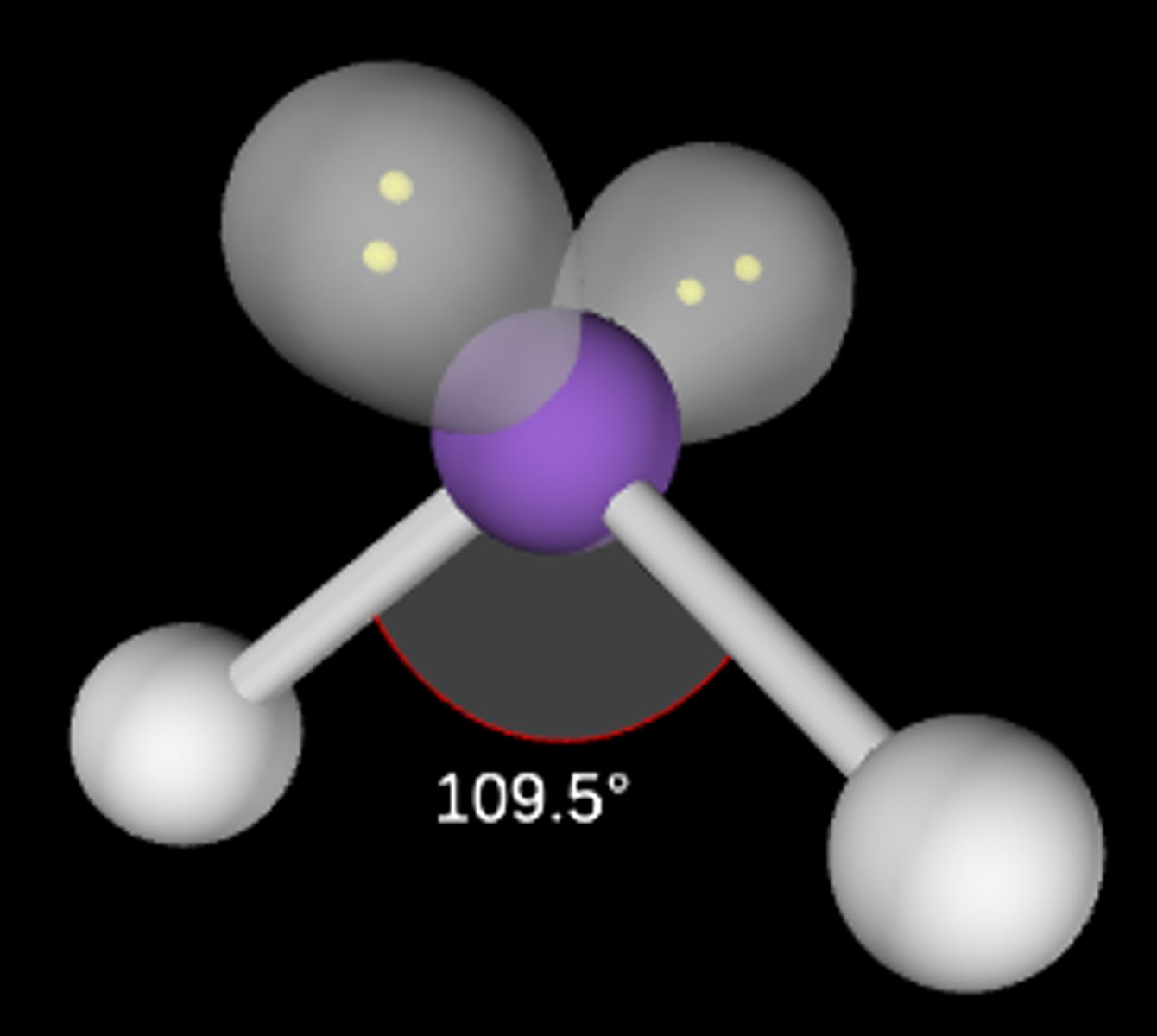
AB2E2
Bent/angular; <109.5°; sp3
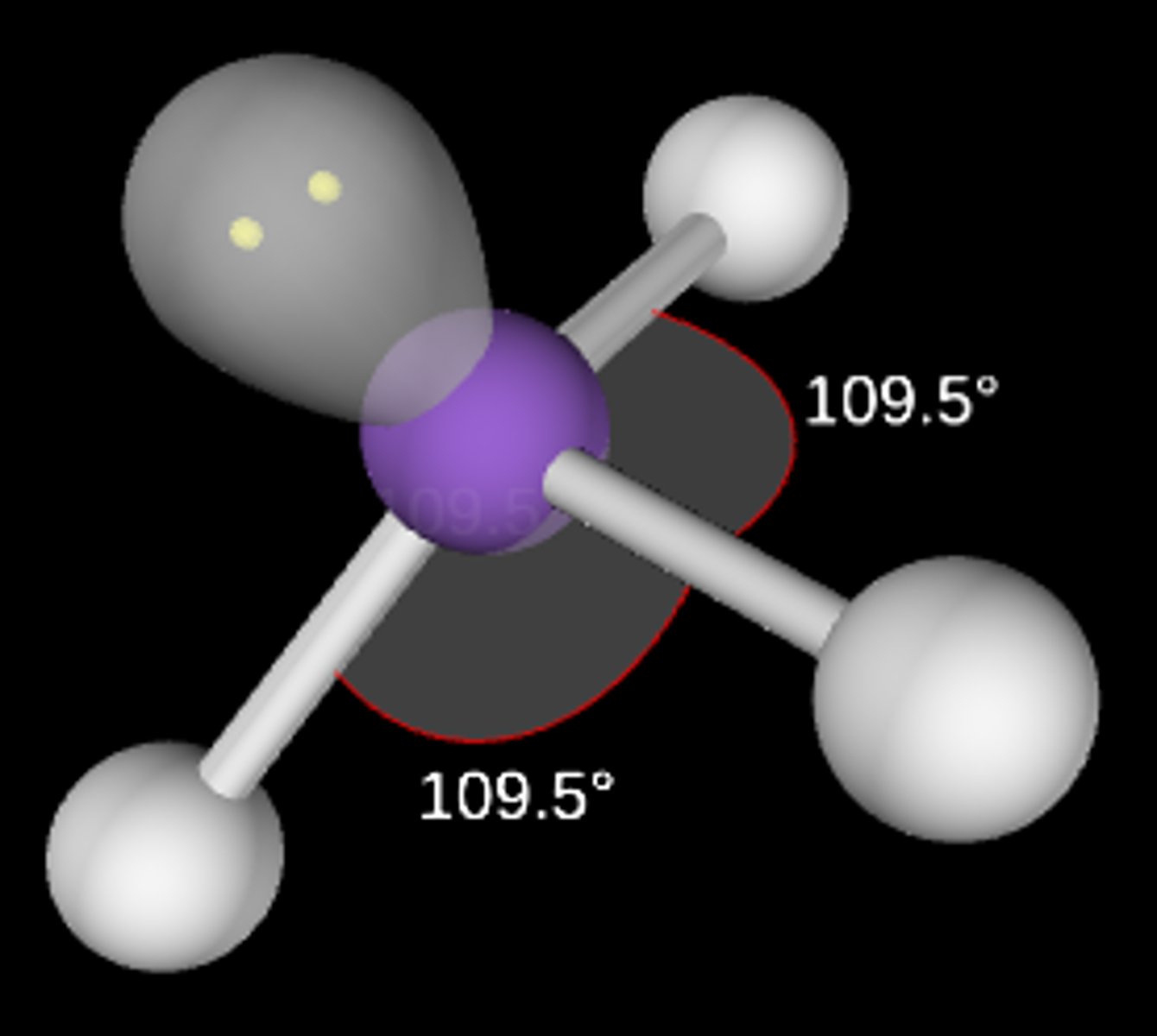
AB3E
Trigonal pyramidal; <109.5°; sp3
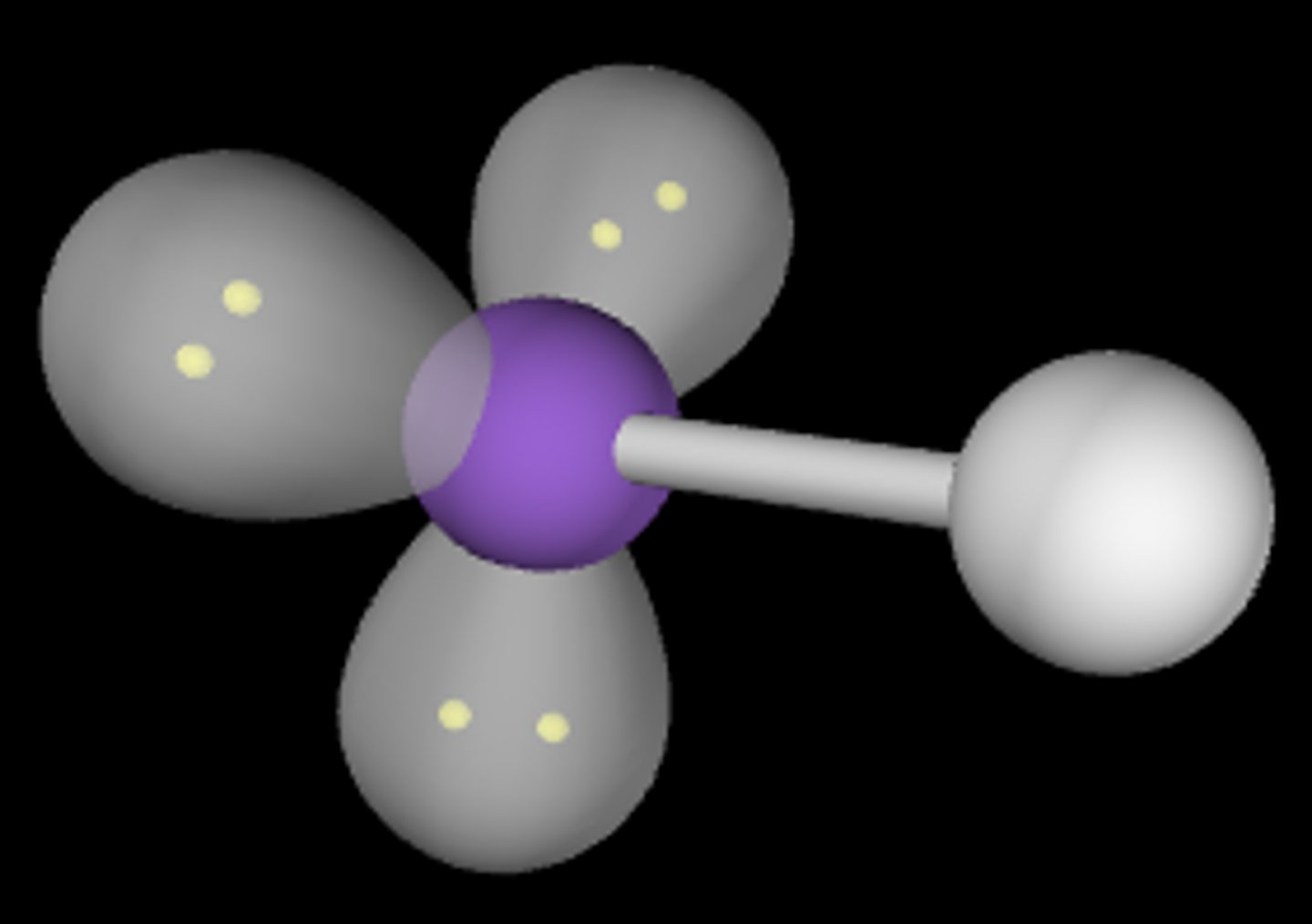
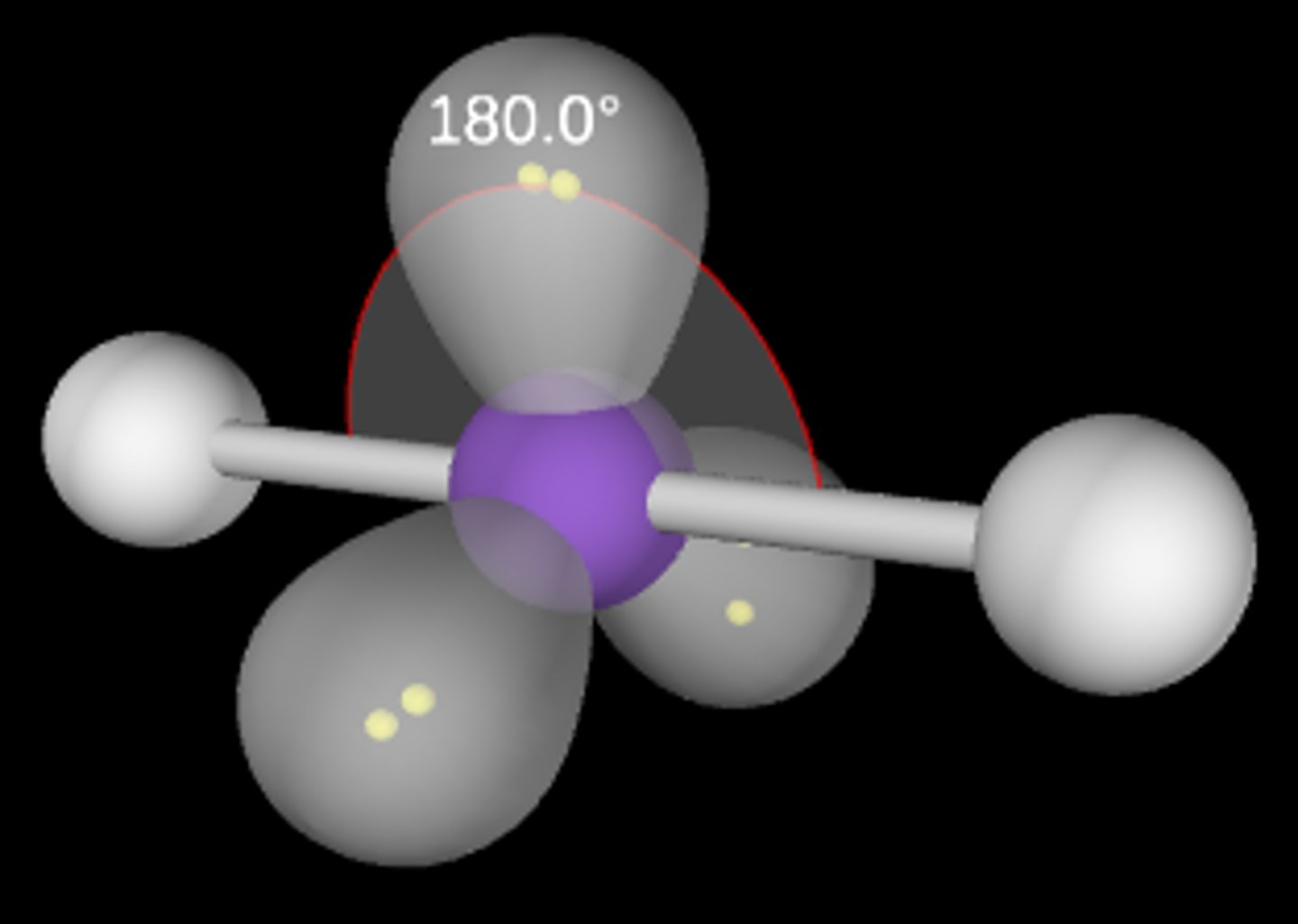
ABE3
Linear; 180°; sp3
AB5
Trigonal bipyramidal; 120° and 90°; sp3d
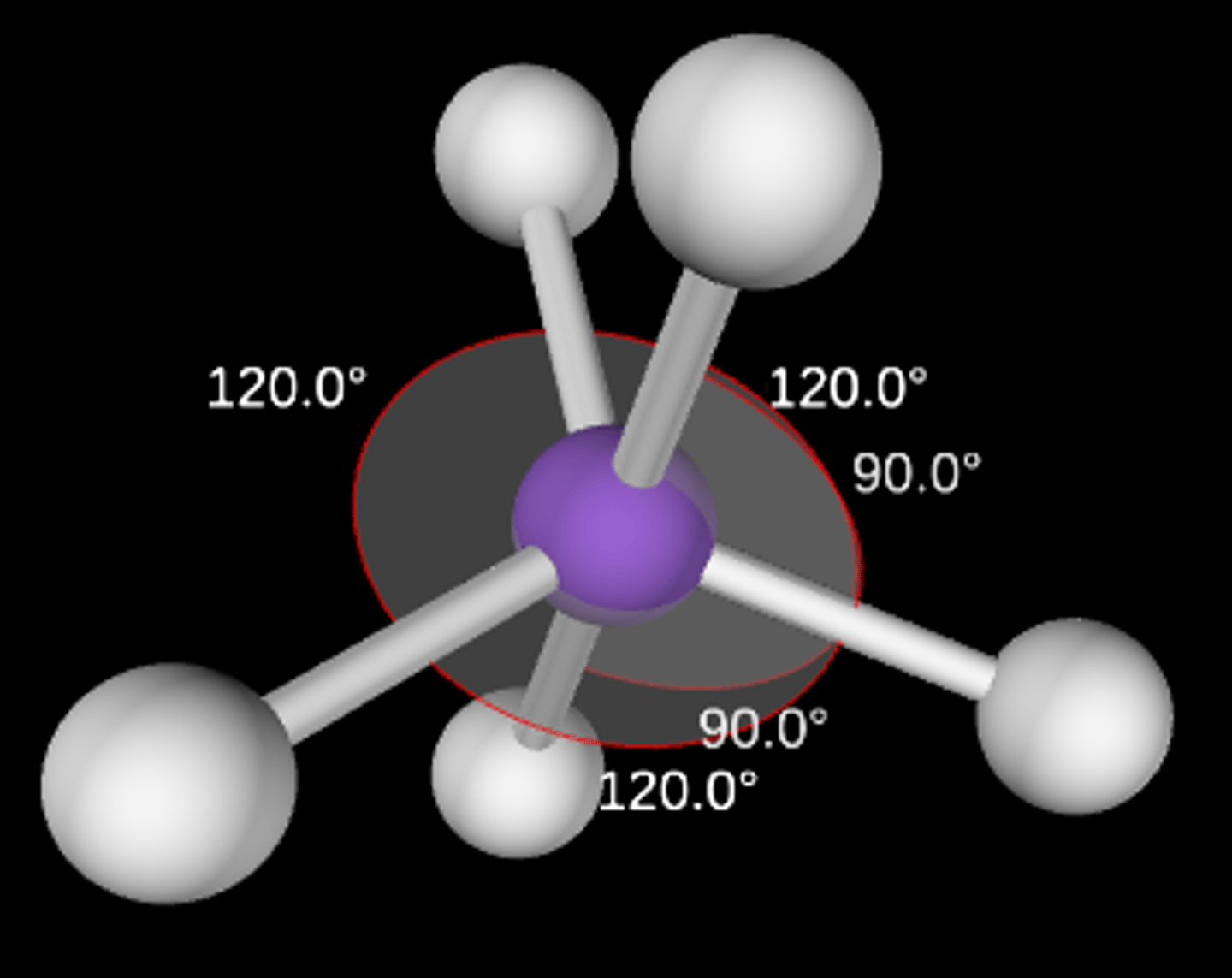
AB4E
Seesaw; <90° and <120°; sp3d
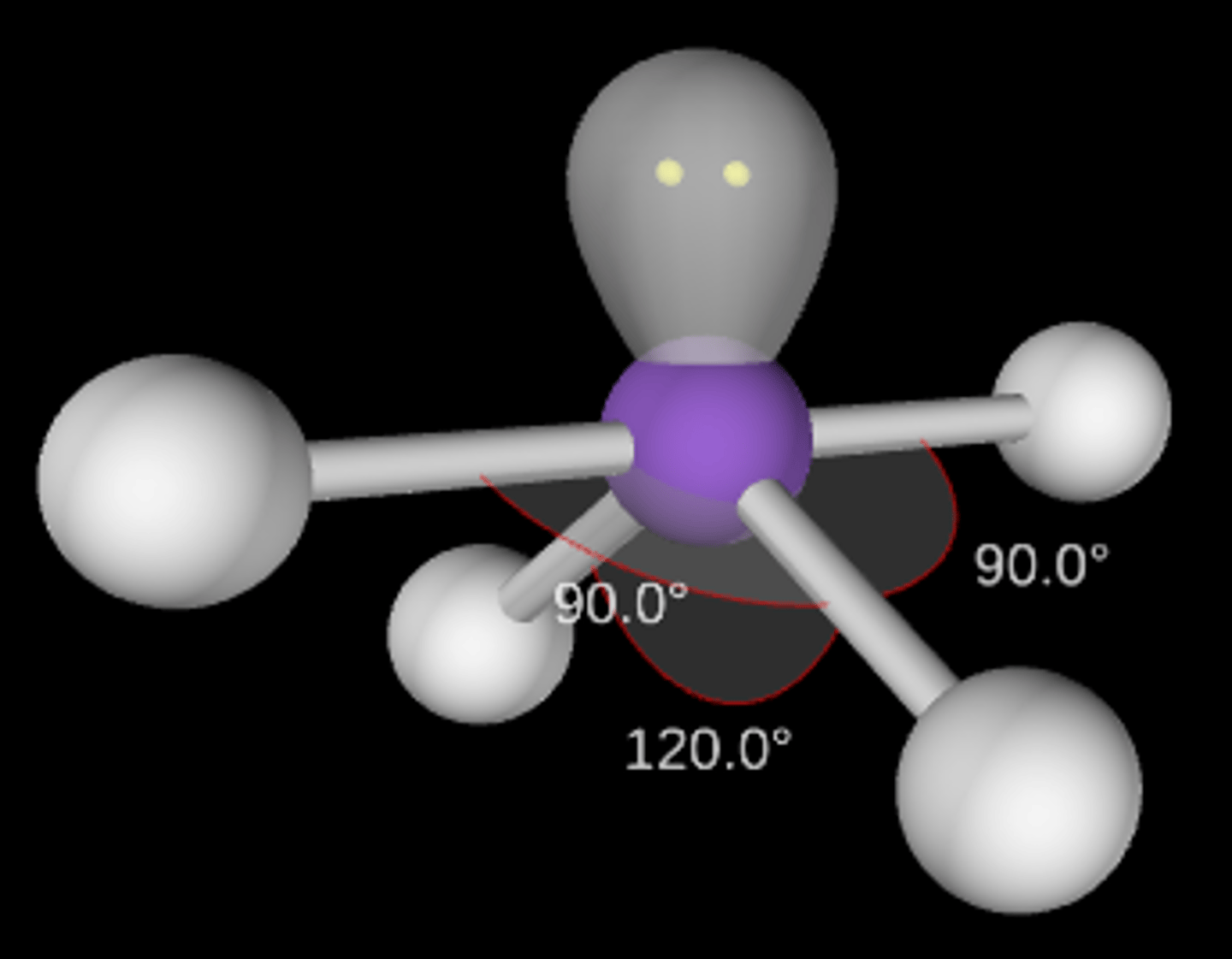
AB3E2
T-shaped; <90°; sp3d
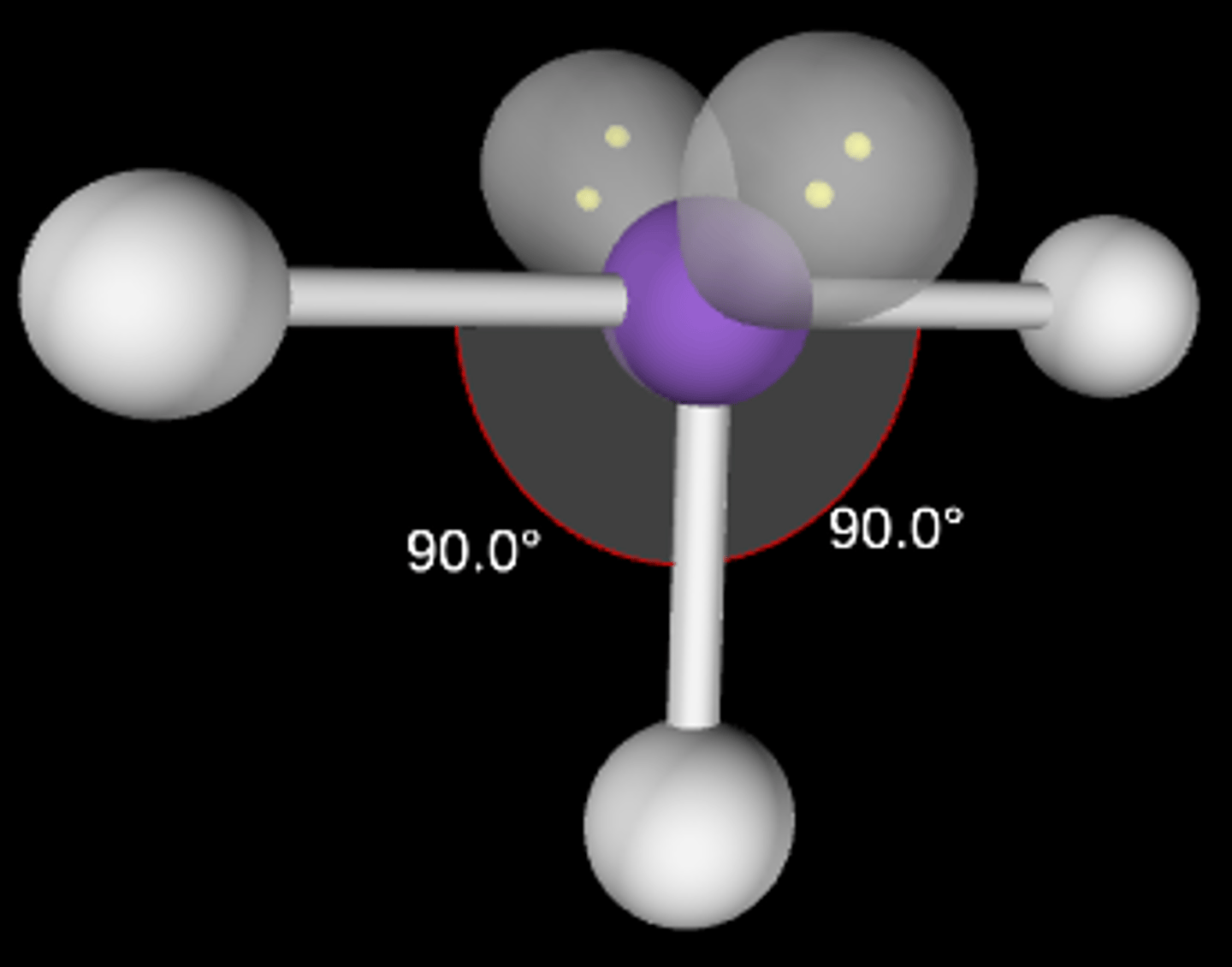
AB2E3
Linear, 180°; sp3d
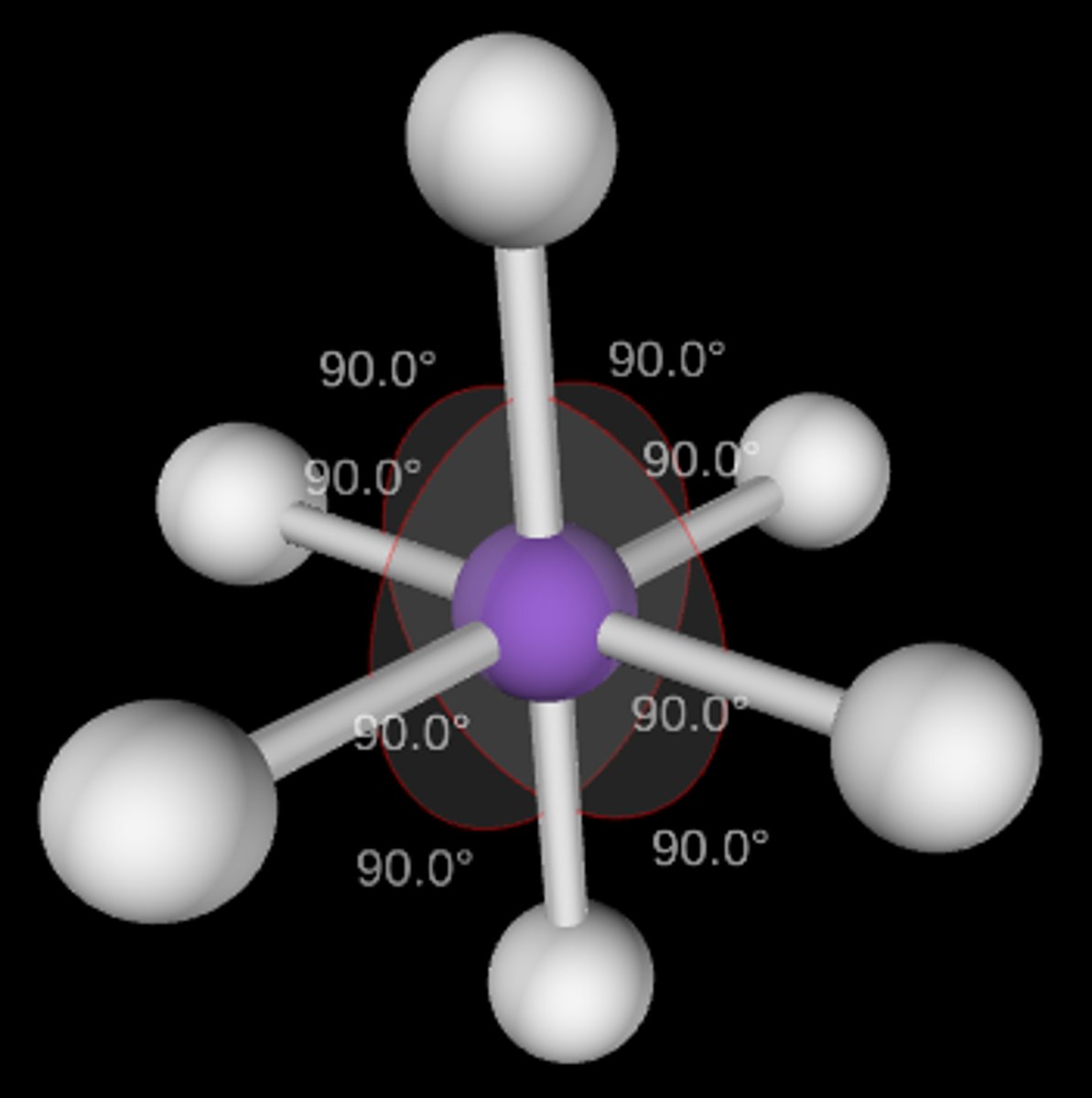
AB6
Octahedral; 90°; sp3d2
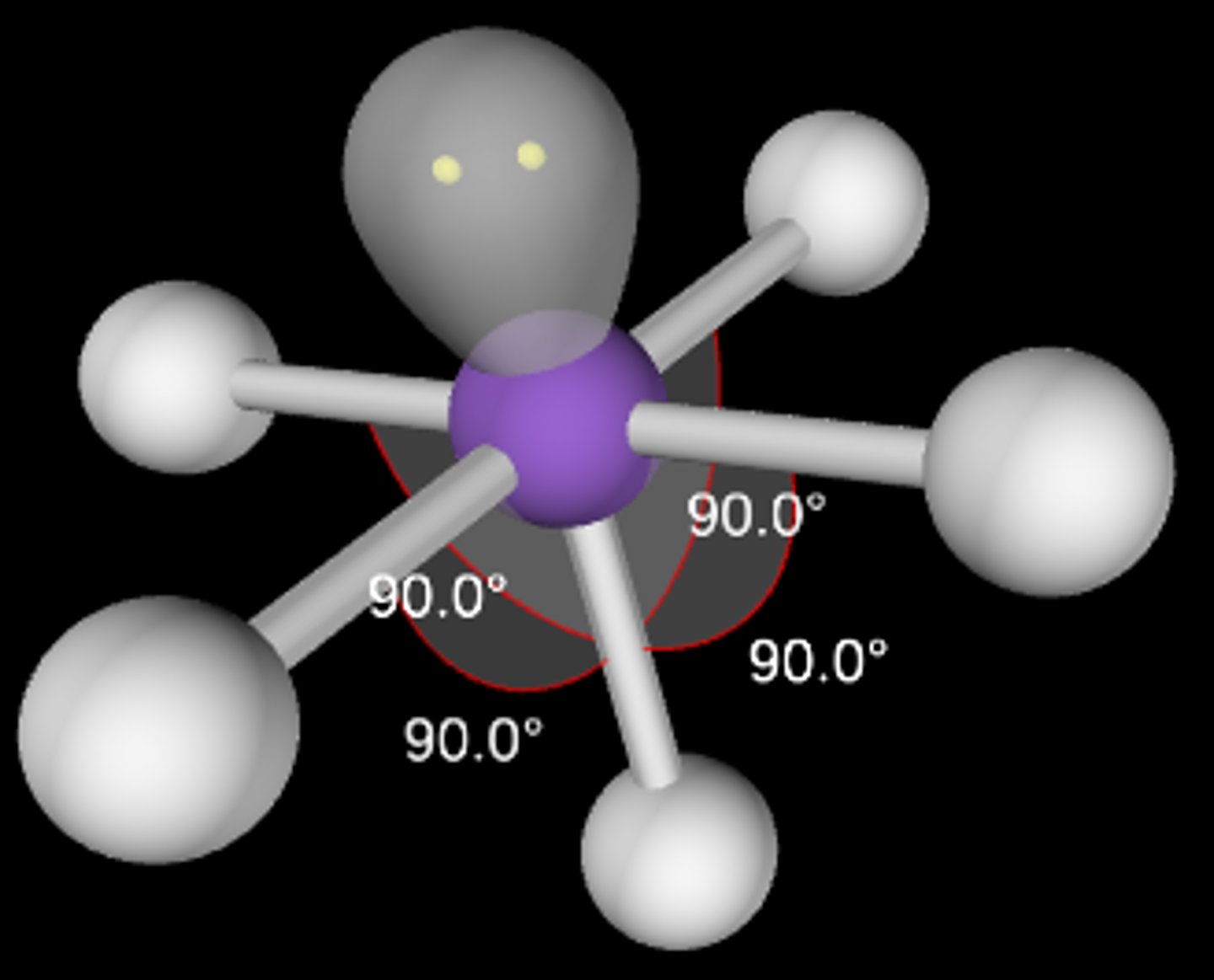
AB5E
Square pyramidal; <90°; sp3d2
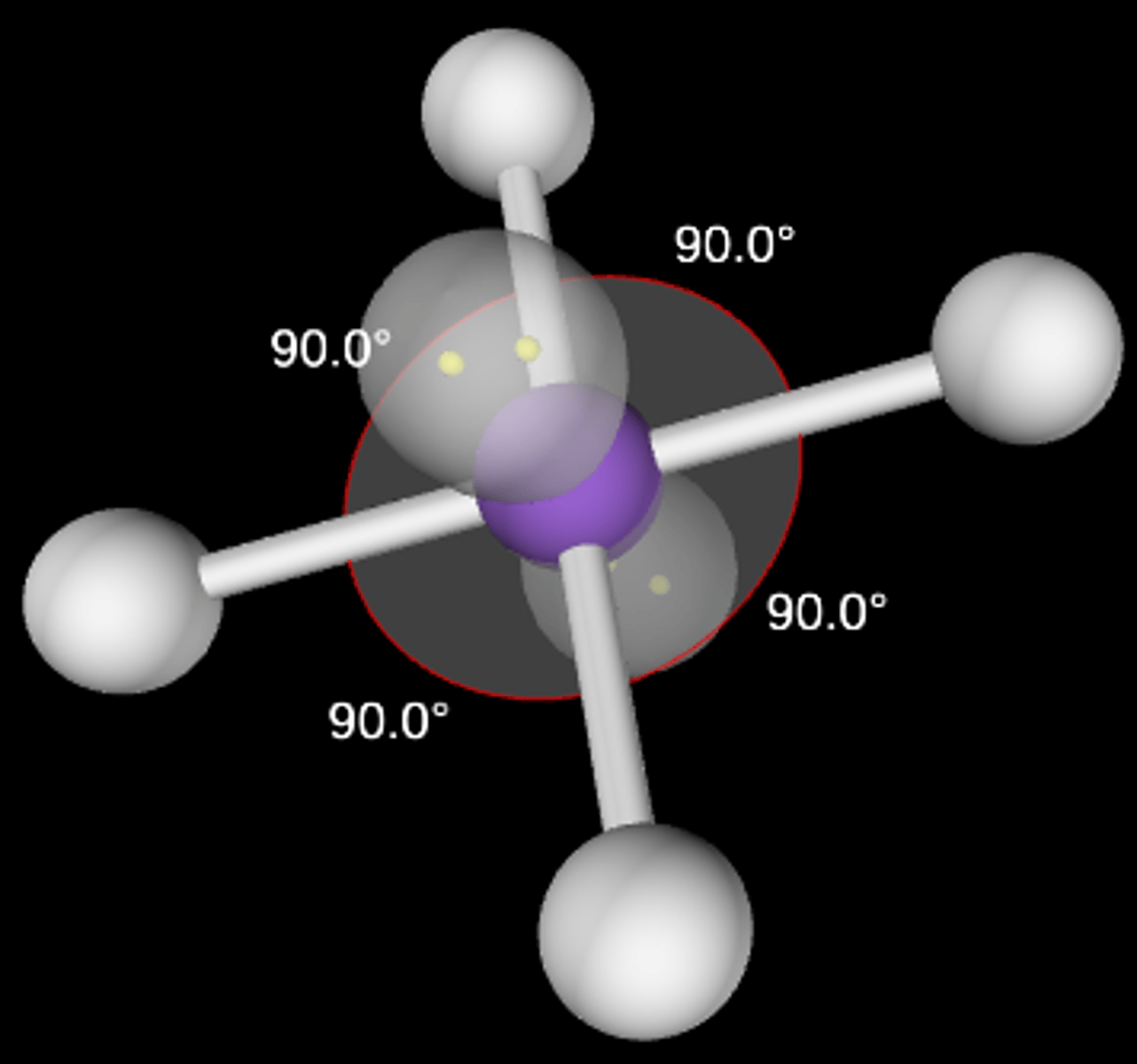
AB4E2
Square planar; 90°; sp3d2
Trigonal
Refers to triangular shape
Hedral
Refers to # of surfaces
Planar
Flat
A in ABE notation represents...
the central atom
B in ABE represents...
the # of atoms the CENTRAL ATOM is bonded to
E in ABE represents...
the # of unshared (or lone) electron PAIRS on the CENTRAL atom