Transition Metals
1/175
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
176 Terms
Why do we see objects as coloured?
We see objects as coloured when they absorb some frequencies of visible light, but transmit or reflect other frequencies of visible light.
For example: "Why is graphite black and calcium carbonate white?"
graphite is black because it absorbs all frequencies of light. Whereas calcium carbonate is white because it reflect of frequencies of light
This is a complementary colour wheel which we can use to predict the colour of objects - learn this
E.g if an object absorbs red light it appears cyan. This is because red and cyan are complementary colours.
E.g if an object absorbs red light it appears cyan. This is because red and cyan are complementary colours.
Explain what is required for a substance to absorb visible light.
There must be room for an electron in one orbital to be excited into another orbital, and the difference between their energy levels must correspond to a frequency of visible light.
E.g the light absorbed will be red because the gap between he energy levels corresponds to the frequency of red light
"Why do most substances not absorb visible light?"
Most of the time visible light isn’t absorbed because the energy level gap is too big
Are azo dyes colourful
Azo dyes are coluourful
Explain why chromium and copper do not follow the usual filling order predicted by the Aufbau principle.
The alpha principle tells us that d block elements fill up 4s and then 3d but chromium and copper are two exceptions with electrons in 3d orbitals. This is because the 4s and 3d orbitals are very similar in energy and when we have a full or exactly half full d subshell the energy of the 3d orbital decreases to be lower than the 4s orbital
Explain the order in which the 4s and 3d subshells are filled in d-block elements.
Aside from the chromium and copper exceptions, electrons fill up the 4s subshell before they start filling up the 3d subshell (becausee its lower in energy). When electrons are lost these electrons are lost from the 4s subshell and not 3d subshell (because the because the 4s orbital becomes higher in energy than the 3d orbital after the 3d subshell begins to fill.)
Define transition metals…
Transition metals are metals which form stable ions with partially filled d orbitals. This electron configuration is part of the reason why trasiotn metals produce colourful compounds, have variable oxidation states and make good catalysts
What is a ligand
A ligand is a molecule or ion with a lone electron pair which forms a dative covalent bond with a transition metal ion. They can be neutral like NH3 and H2O or they can be negative lie Cl-
What is the coordination number
The coordination number is the The number of dative covalent bonds to each metal ion. The size of each logan is one factor which determines the coordination number
Complex ion
When we have ligands around an ion.
"How do we show the charge of complex ions?"
We show each complex ions overall charge outside of a pair of square brackets. E.g cobalt (Cu3+) has a charge of 3+ and NH3 (amonia) has no charge so the overall charge is 3+
"Why does cisplatin have no overall charge and not require square brackets?"
Cisplatin has no overall charge because platinum has a 2+ charge. The 2Cl - molecules balance out this 2+ charge and NH3 is neutral/no charge. This means we dont need to draw square brackets around it
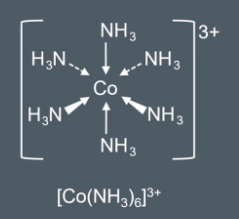
How to draw draw the formula of complex ions
We can draw the formula of complex ions by suing square bracket and round brackets.
Write the complex ion formula
Write the ligand molecule formula in square brackets and then add the number of ligands outside the square brackets. Encase all of this in a square bracket and add the overall charge
"What charges can complex ions have?"
Complex ion can have a overall charge that Positive negative or neutral.
"How are complex ions named?"
Complex ions are named according to the number and type of its ligands, the metal ion and its oxidation state
Draw hexaaquacopper (II]
In exams When asked to draw a complex ion
Make sure to have wedges for bonds coming towards you and dashes for bonds that are away. (You can add arrows to the dashes and lines to indicate it is a dative covalent bond but this is optional).
We need to make sure that the bonds begin at the atom with the lone pair (so oxygen for water and nitrogen for ammonia).
Add square brackets and include the overall charge of the complex ion
Drawing tetrahedral complex ions (4 ligands)
Drawing linear complex ions with 2 ligands (linear shape)
"What is the shape and structure of Tollens' reagent?"
Tollens reagent is a complex ion with 2 ammonia ligands so it has a linear shape that we draw like above.
Drawing octahedral, tetrahedral and linear complex ions
"What is the shape of complex ions with 4 ligands?"
Some complex ions with 4 ligands are tertahedral but some a square planar. Remember that complex ions of nickel, palladium and platinum are square planar shapes and not tetrahedral shapes
Some complex ions with 4 ligands are tertahedral but some a square planar. Remember that complex ions of nickel, palladium and platinum are square planar shapes and not tetrahedral shapes
E.g
"How does stereoisomerism apply to square planar complex ions?"
Stereoisomerism applies to complex ions.
If there are two identical ligands on the same side in a square planar complex ion, then we give them the letter Z or the word Cis as a prefix.
If the two identical ligands are on opposite sides/ diagonal to each other in a square planar complex ion, then we give them the letter E or the word trans as a prefix.
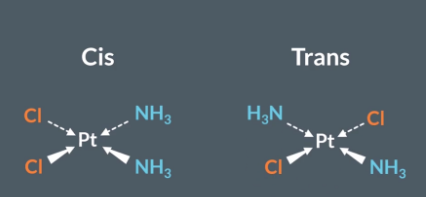
What complex ions can display cis/trans isomerism
For complex ions to display cis/trans stereoisomers, they must be square planar or Octahedral but only if they have 2 identical ligands
(tetrahedral can’t display this because all the ligands are next to each other)
"What is the difference between cis and trans isomers in square planar complexes?"
If the two identical ligands are on opposite sides they are trans isomers. But if they are next to each other or one way away from each other they are cis
When light appears one colour e.g cyan it could be because of:
monochromatic ion
polychromatic ion
"What is monochromatic cyan light?"
monochromatic cyan which is where cyan is composed of one frequency of light (monochromatic cyan light)
"What is polychromatic cyan light?"
Polychromatic cyan light where cyan is composed of multiple frequencies of light e.g blue, cyan and green or blue, cyan, green, magenta and yellow light
How to distinguish between monochromatic and polychromatic light
We can use colour filters to distinguish between monochromatic light and polychromatic light. For example if we take polychromatic cyan light and shine it through a filter some light will be detected. Whereas if shine monochromatic cyan light though a green filter none of the cyan light would detected
What is d orbital splitting
When d orbitals split into two different energy levels
Why are transition metals colourful
Transition metals have d orbital electrons. In complex ions, the ligands interact with the d orbitals, causing some of the orbitals to increase in energy level and others to decrease (d orbital splitting).
This creates a small gap in energy levels, which corresponds to one of the frequencies of visible light.
So light of that colour excites an electron to a higher energy d oritbtal and is absorbed
Why isn’t hexaaquazinc (II) colourful?
Because the d orbitals are completely full
To be colourful metal ions need to have electrons in d orbitals which split in energy level and their need to be space for a electron to be promoted from a lower energy orbital to a higher energy orbital
There aren't any stable zinc ions with partially filled d orbitals so it isn't a transition metal.
Summary of How colour airse
Colour airses when electrona re excited form one orbital to another higher energy orbital and the gap between them corresponds to frequency of light in the visible speocturm
Factors that affect d orbital spliiting
"What is the ground state of an atom?"
An atom is in its ground state when all of it electrons are in its lowest possible energy level
"What is the excited state of an atom?"
An atom is in its excited state when at least one of its electrons is not in its lowest posible energy leve
"How is the energy gap related to the frequency of light absorbed?"
Difference in energy levels is proportional to frequency of light absorbed. So the larger the gap in different energy levels the larger the frequency of light absorbed
"How does the energy gap affect the color of light absorbed?"
High frequency light is close to the blue end of the visible light spectrum. So the bigger the energy level light the bluer the light absorbed
Equation for Calculating energy level gap/ change in energy from the frequency
Equation for the change in energy = planck's constant (value given in exams) * frequency
This equations need to be remembered
H = planks constant
V = frequency
Multiply the value of H by V ( frequency of the blue light)
What color light would this compound absorb
Divide E by H we get 4.5*10^14 which is the frequency of red light (when we compare it to a table fo frequencies of light). The red light would be absorbed. SO we would see the colour as cyan as cyan is red complementary colour
Calculating the energy level gap/change in energy from the wavelength
wavelength is in metres
1nm = 1 × 10 9 m
C= speed of light (given in the exam). WE NEED TO MEMORISE THIS EQUATION
350 nm into metres
wavlentgth of light/ value of V (frequency of light) to identify colour
relating frequency to energy level gap
You may be asked to calculate the energy level gap from the frequency or the wavelength
So you need to remember both equations. But you will be told the value of planks constant (h) and the speed of light ©
State and explain how the oxidation state of a transition metal ion affects the color of its complexes.
One factor which affects the colour of a transition metal complex is the oxidation state of the metal ion.
Higher oxidation states mean more d orbital splitting, and so higher frequencies of light are absorbed.
However this affect is usually difficult to see because other frequencies of light are often absorbed as well
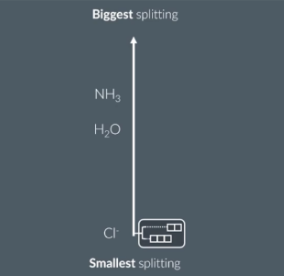
Explain how the type of ligand affects the color of a transition metal complex.
Different ligands cause different degrees of d orbital splitting. So NH3(ammonia) would absorb more blue light whereas Cl would absorb more red light.
The diagram above ranks the ligands based on the amount of splitting they cause
For example
The molecule appears magenta which means it is absorbing green light. This is because it has ammonia ligand which cause the biggest d orbital splitting
Red end of the spectrum
"How does coordination number affect light absorption in complex ions?"
Complexes with higher coordination numbers absorb higher frequencies of light (e.g bluer light) because of greater d orbital splitting. So a complex ion with 6 coordination number will absorb blue light giving us colours closer to red, yellow or green (complementary colours)
(more ligands = more splitting)
Outline the trend between concentration and light absorbed
The more concentrated a solution the more light it will absorb. E.g a more concentrated magenta solution will absorbs more green light
How do we use colorimetry to analyze coloured samples
E.g to analyse a sample of hexaaquacopper II which has a cyan colour we would shine light through a red filter (complementary colour) and use a detector to measure the absorption. We would then compare this value to samples of hexaaquacopper II with a known concentration (E.g 20mol/dm-3 sample that absorbs 20% light etc). Draw a calibration curve by plotting the known concentrations. Use the calibration curve identify the concentration of unknown sample.
How do we analyze a sample of magenta?
To analyze a sample with a magenta colour we would use green light because green and magenta are complementary colours.So a magenta solution would absorb green light most strongly. The more concentrated the magenta solution the more green light it will absorbs.
Define ligand substitution reaction
When at least one ligand replaces another, we call this a ligand substitution reaction
What happens to the color of a solution when a water ligand is substituted with an ammonia ligand?
here we have substituted a water ligand with ammonia ligand. Ammonia ligand cause more d -orbital splitting. This means a slightly higher frequency of light is absorbed making the solution appear less cyan and more deep blue
Complete ligand substitution
All of the ligands have been replaced
Incomplete ligand substitution
Incomplete ligand substitution because 2 of the original ligand are still left. Overall equation is shown above. The reaction is reversible
"What happens during ligand substitution reactions?"
In ligand substitution reactions the coordination number might change or stay the same. The overall charge might change or stay the same.
For example
Here we have a ligand substitution reaction where 6 H2O molecules are replaced by 4 Cl- molecules. This is due to Cl- size which is too big for 6 to fit around the copper ion
Also here 6 neutral water ligands are replaced by 4 negative Cl ligands so the charge decreases from 2+ to 2-
What is a bidentate ligand
Certain molecules are long enough and flexible enough to form two dative covalent bonds with a metal ion
How do we draw bidentate ligands and what is the coordination number of this bidentate ligand?
Either way of drawing bidentate ligands is acceptable in the exam. This complex has 3 bidentate ligands and 6 dative covalent bonds so it has a coordination number of 6
"What is 'en' in complex ion chemistry?"
We can refer to this bidentate ligand as en in formulas and equation for short. En is a neutral ligand
when you need to write the structural formula :
Draw the complex that would be produced by adding an excess of ethane-1,2-diamine (en) to hexaaquacopper (II).
Draw the (Cu(en)3)2+ ligand
Draw the central metal ion
To draw the octahedral shape. Draw two normal bonds, two edge bonds and two dotted bonds
Draw the 3en ligands
Draw square brackets and add the charge
Note than en is a neutral ligand (e.g if we substitute water ligands for en ligans the oevrll charge doesnnt change
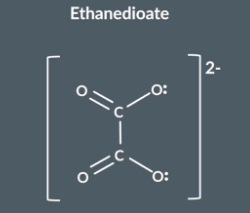
"What is ethanedioate and how does it act as a bidentate ligand?"
This is structure of ethanedioate another bidente ligand. Its acts a bidenta ligand when the lone pairs on the negatively charged oxygen atoms form a cooridante bond with a metal ion
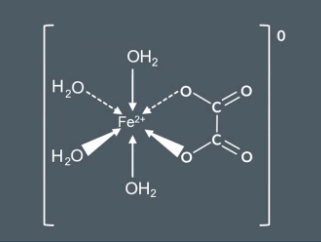
![<p>if one ethanedioate ligand replaces two of the water ligands on [Fe(H2O)6]2+,we end up:</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/90db38c9-4212-4e82-b88e-bf1dc7253c53.png)
if one ethanedioate ligand replaces two of the water ligands on [Fe(H2O)6]2+,we end up:
It has a coordination number of 6 and a charge of 0 because the 2- charge of ethanedioate ligand cancels out the 2+ charge of Fe
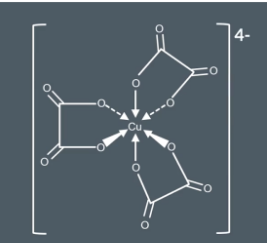
Draw the complex that would be produced by adding an excess of ethanedioate to hexaaquacopper (II).
6 neutral water ligands would be replaced by 3 ethane dioate (2-) ligands. Cu has a 2+ charge and the 3 ethanedioate ligands have a 6+ charge giving the overall molecule a 4- charge.
what is the full equation for the reaction of adding an excess of ethanedioate to hexaaquacopper (II).
This is the full equation for this reaction
Learn the formula of these two bidente ligands
bidentate ligands and optical isomerism
Compounds like the two above are optical isomers because they have mirror images which are non super imposable
The chelate effect
Multidentate ligands (right) replace monodentate ligands (left). (e.g when a bidentate ligand replaces a monodentate ligand). This is called the chelate effect. Chela is greek for claw which k looks like a multidentate ligand
In your answer you should write about the total number of particles in the reactant and the product. E.g here
There are 4 particles in the reactant and 6 particles in the product; entropy increases over the course of the reaction so the reaction is likely to proceed.
ALWAYS USE THE TERM PARTICLES AND NOT MOLECULES/IONS BECAUSE MOLECULES DOESN'T COVER EVERYTHING
A full answer would look like this - memorise this structure
Explain why the reaction proceeds with reference to the chelate affect?
Multidentate ligands (rigth) replace monodentate ligands (left).
This is because entropy is likely to increase if there are more particles in the products (x) than in the reactants (x).
This is known as the chelate effect.
what is this ligand known as
This ligand is known as EDTA
It can form 6 dative covalent bonds with a metal ion.
Its charge is 4-
It can react quickly with any heavy metals in the body because of the chelate effect.
Why is EDTA a extremely useful treatment for heavy metal poisoning
The numer of particles in the product (7) is more than the number of particles in the reactant (2). This results in large increase in entropy which drives the reaction forward.
SO when a patient with heavy metal poisoning is treated with EDTA the EDTA quickly reacts with lots of the heavy metal ions and this prevent the heavy metal ions from causing damage to the body because the heavy metal ion always stays bonded to the EDTA as a result of the chelate effect. (Multidente vs bidente --- particles -- entropy)
Describe how haemoglobin transports oxygen in the body, including the role of the Fe²⁺ ion in the haem group.
Harem consits of a mulitdente ligand which forms 4 dative covalent bonds to a central Fe2+ (iron II ion). Once a protein called globin joins the complex an oO2 molecule can form a dative covalent bond to the Fe2+. when the blood reached an area whci needs O2 an O2 ligand is replaced by a H2O ligand. This allows the body to transport O2 to areas where it is needed for respiration
(c) Haemoglobin can release oxygen in areas where it is needed. Explain the ligand substitution reaction that occurs when oxygen is released.
When haemoglobin reaches an area of the body with low oxygen concentration, the O₂ ligand is replaced by a H₂O ligand in a ligand substitution reaction. This process ensures that oxygen is released where it is needed for respiration.
Carbon monoxide poisoning occurs when CO binds to haemoglobin. Explain how this affects the transport of oxygen and carbon dioxide in the body.
Oxygen, water and carbon dioxide form UNstable bonds with hemoglobin. However CO (carbon monoxide) forms a stable bond with hemoglobin (stronger ligand and CO-Fe bond is stronger). This means that CO molecules bond to hemoglobin permanently preventing it from transporting O2, H2O and CO2 around the body
what is a catalyst
A catalyst is a substance that increases the rate of a reaction without being used up in the reaction.
"What is a homogeneous catalyst?"
Catalysts which are in the same state as the reactants are called homogeneous catalysts.
"What is a heterogeneous catalyst?"
Catalysts which are in a different state to the reactants are called heterogeneous catalysts.
"How does iron act as a catalyst in the Haber process?"
In the Haber process, solid iron acts as a heterogeneous catalyst, providing a surface for nitrogen and hydrogen to adsorb and react to form ammonia. This lowers the activation energy, increasing the reaction rate. The part of the catalyst surface where reactants adsorb is called the active site.
"How is catalyst efficiency maximized in industrial reactions?"
To maximize efficiency, catalysts are often used as powders or thin coatings on substances with large surface areas, like porous ceramics, to maximize the number of active sites. This support media reduces the amount of catalyst needed, lowering costs while maintaining high surface area. Only heterogeneous catalysts provide a solid surface for adsorption and reaction.
"How can the effectiveness of a heterogeneous catalyst be increased?"
Heterogeneous catalysts can provide a(n) active site for the reaction.
The surface area of a catalyst can be increased by turning it into a powder, or by applying it as a coating onto a support medium .
"How does vanadium pentoxide act as a catalyst in the contact process?"
In the contact process, vanadium pentoxide acts as a heterogeneous (transition metal) catalyst by providing active sites for the reactants to adsorb on to. Vanadium pentoxide uses the variable oxidation states of vanadium to provide an alternative reaction route with lower activation energy .
Explain what is meant by “catalyst poisoning”.
Catalyst poisoning occurs when impurities bind to a catalyst’s active sites and prevent it from functioning.
This reduces the number of active sites which makes the catalyst less effective over time.
Catalyst poisoning in the contact process to make sulfuric acid
In the contact process to make sulfuric acid the sulfur dioxide contains impurities and to prevent catalyst poisoning form happening scientists remove impurities front the sulfur dioxide supply. E.g dust in sulfur dioxide is removed by spraying water over the SO2 gas.
Example of a homogeneous transition metal catalyst
Example of a homogeneous transition metal catalyst
Example of a homogeneous transition metal catalyst
"Why can't iron provide active sites in homogeneous catalysis?"
Iron cant provide active sites because being a homogenous catalyst it cannot be a solid. Iron is regenerated (When a catalyst reacts in one step of the reaction, but returns to its original state in a later step )
Explain what is meant by the term “autocatalyst”.
A catalyst which is one of the products of the reaction it catalyses.
Why can the Mn2+ be considered an autoocatalyst?
In this reaction the product which is an Mn2+ ion acts as catalyst because its positive manganese ion can attract the negative reactions so it lowers the activation energy and speeds up the reaction. The MN2+ ions will get regenerated at the rend of the reaction