Alcohols
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
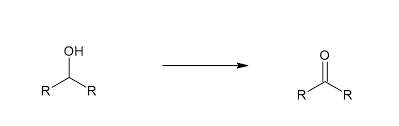
Reduction or Oxidation?
Oxidation
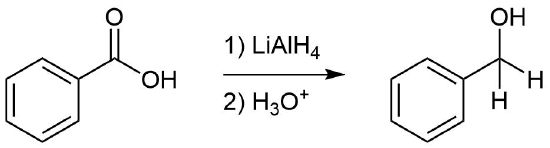
Reduction or Oxidation?
Reduction
If a chemical with a double bond and an alcohol gets reduced by H2 & Pt, what component(s) get reduced?
Both the alcohol and double bond
What is the mild reducing agent, and what can it NOT reduce? What conditions are reactions done in?
NaBH4, carboxylic acids, Alcohol or water as solvent
What is the strong reducing agent and what conditions are reactions done in?
LiAlH4, anhydrous ether
If you have a chemical with a carbonyl group and an alkene, which is to be reduced by LiAlH4, which component(s) get reduced?
Just the carbonyl group
What type of alcohol cannot be oxidized?
Tertiary
What are the strong oxidants?
Potassium/ Sodium dichromate with sulfuric acid, chromic acid
What results if a primary alcohol is oxidized by potassium dichromate?
Carboxylic Acid
What results if a secondary alcohol is oxidized by potassium dichromate?
Ketone
What are the mild oxidants?
PCC, PDC
What results if a primary alcohol is oxidized by PCC?
Aldehyde
What results if a secondary alcohol is oxidized by PCC?
Ketone