Nuclear Physics Merged Flash Cards (Feb 22 10:06 pm)
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
The element stays the same but loses energy.
Fluorine, ¹⁹ 9F is the element with atomic number 9, and its nuclear symbol indicates it has a mass number of 19.
What did Henri Becquerel discover about radiation emitted from uranium salts?
He discovered that uranium salts emitted rays that darkened photographic plates.
Distinguish between the terms “mass number” and “ atomic number".'“
Mass Number= Proton Amount + Neutron Amount. Atomic Number= Number of Protons
How do various isotopes of an element differ?
They differ with the amount of neutrons within each isotope.
What two rules relate to mass numbers and atomic numbers in a nuclear equation?
The sum of the mass numbers does not change.
The sum of the charges in the nucleus does not change.
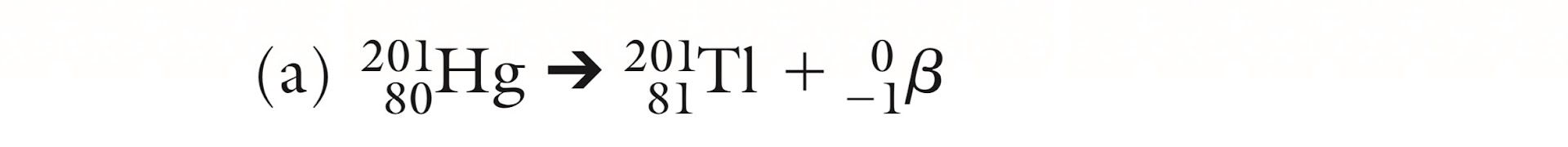
Is this Alpha, Beta or Gamma decay?
Beta decay
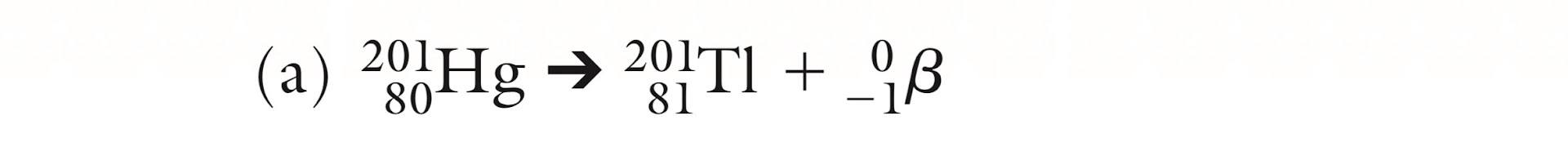
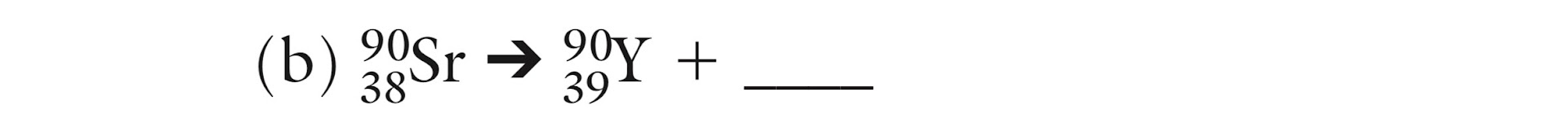
Is this Alpha, Beta or Gamma decay?
Beta decay
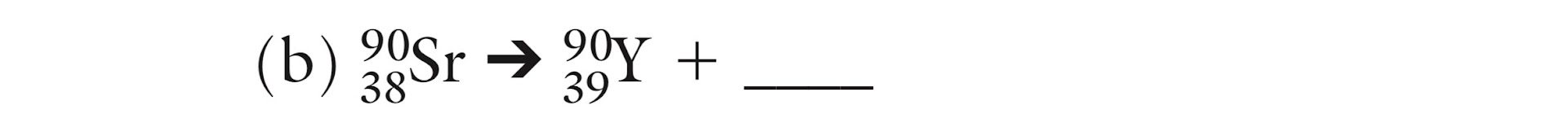
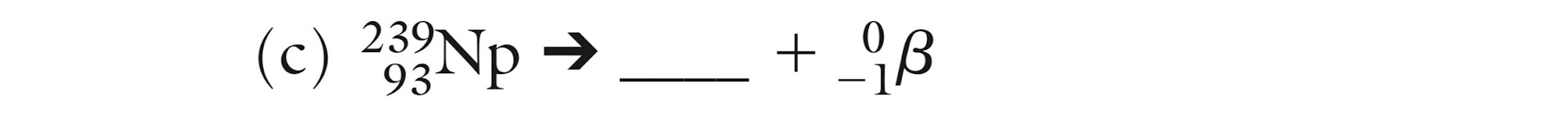
What is the missing isotope?
239/92 U
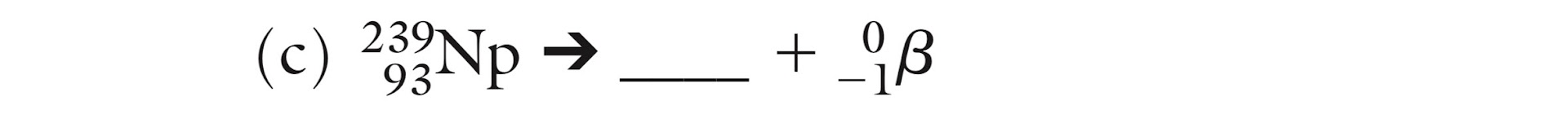
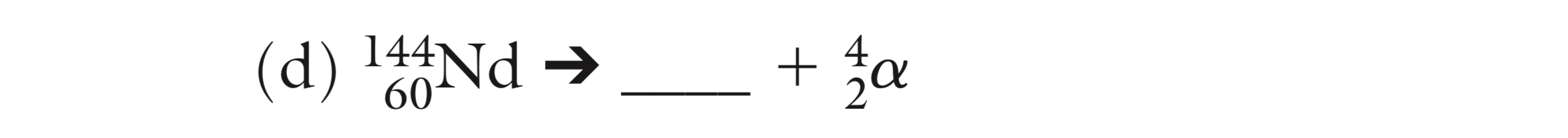
What is the missing isotope?
140/58 Ce
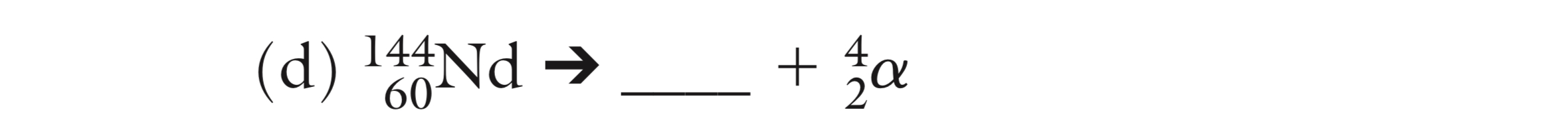
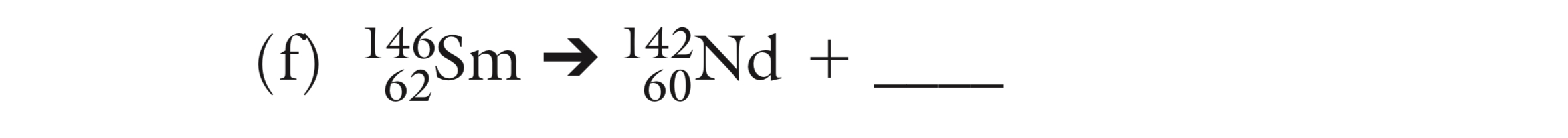
Is this Alpha, Beta or Gamma decay?
Alpha Decay
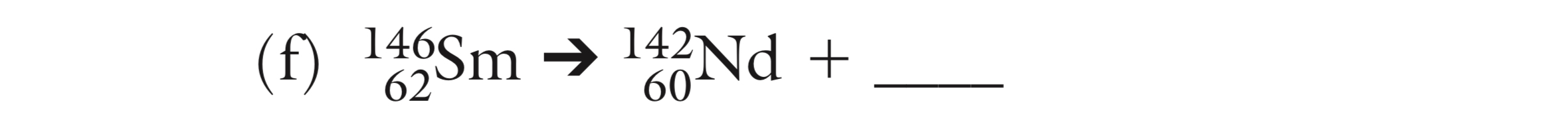
How many neutrons are in Potassium-39?
20
How many neutrons are in Potassium-40?
21
How many neutrons are in Potassium-41?
22
How many protons are in Potassium-39?
19
How many protons are in Potassium-40?
19
How many protons are in Potassium-41?
19
Why was the discovery that uranium salts emitted radiation a surprise to scientists?
The discovery that uranium salts emitted radiation was a surprise because scientists didn't know that substances could emit energy without any external source, like heat or light. (happend naturally)
What did Marie Curie call the process by which some materials give off radiation such as X rays?
Radioactivity
What is it meant by the term "isotope?"
Isotopes are different atoms of a particular element that have the same number of protons but different numbers of neutrons.They have the same atomic number but different mass numbers.
What do all isotopes of the same element have in common?
They have the same symbol
How do isotopes of the same element differ?
They differ by mass number and different # of neutrons in the nucleus.
What information about the nucleus of an isotope is given by it's mass number?
The mass number of an isotope tells you the total number of protons and neutrons in its nucleus. It helps distinguish between different isotopes of the same element.
What is the length of one half-life of Carbon-14?
5730 years
How many half-lives have passed when there is ¼ of the original amount of carbon-14?
Two half-lives have passed, as each half-life reduces the amount by half, resulting in one quarter of the original amount.
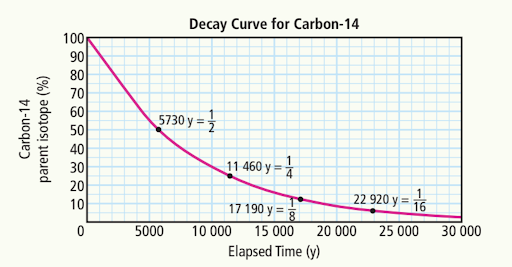
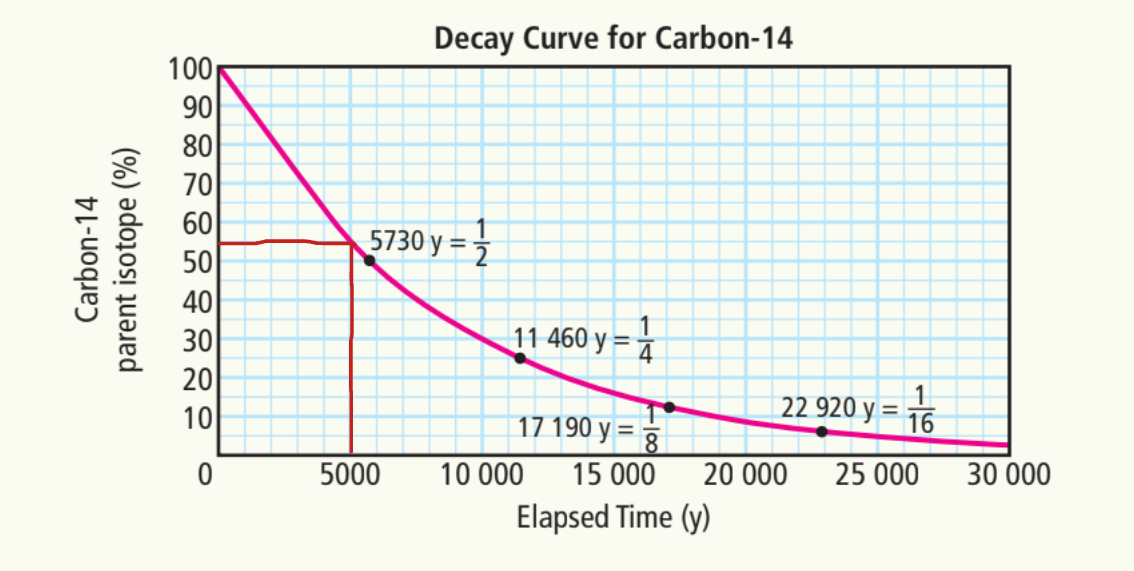
Estimate the percentage of carbon-14 after
A) 5000 years
55%
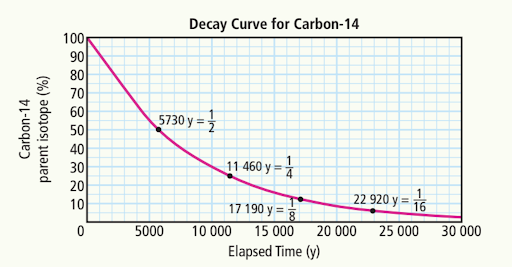
B) 10,000 years
30%
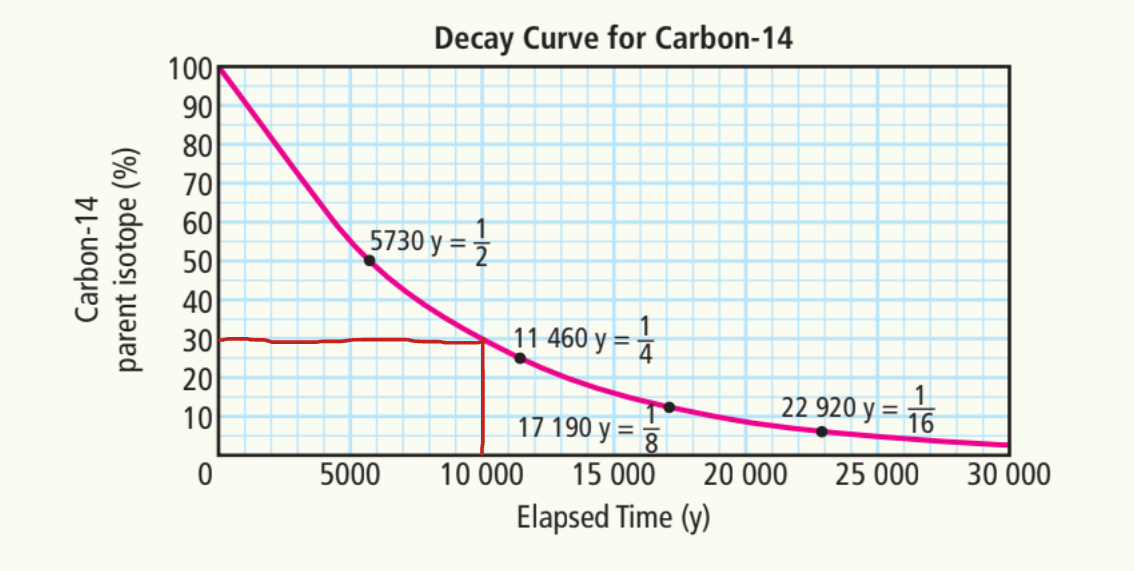
C) 20, 000 years
~8%
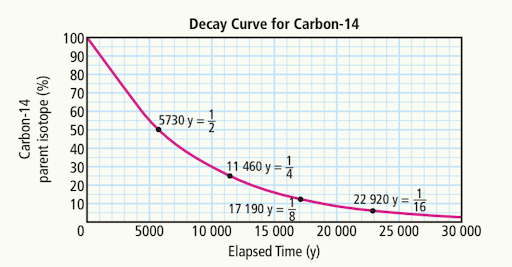
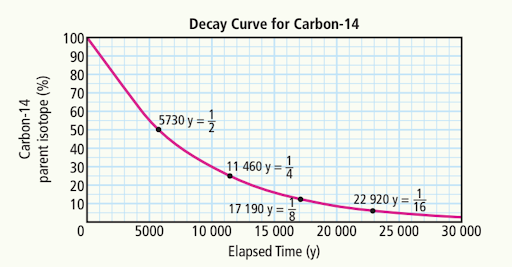
Estimate the time elapsed when the amount of carbon-14 remaining is:
a) 70%
a) ~3000 years
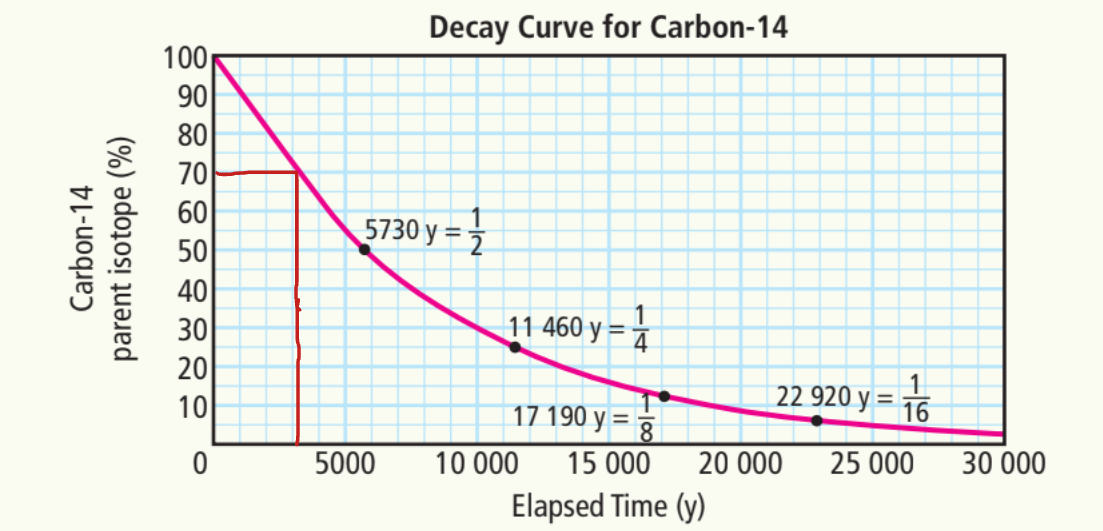
b) 40%
~7500 years
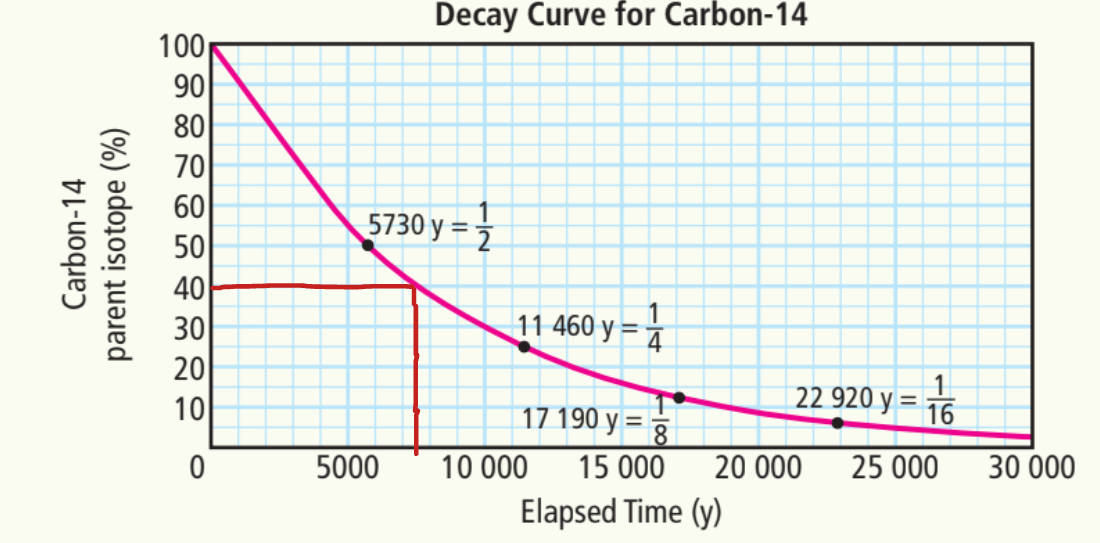
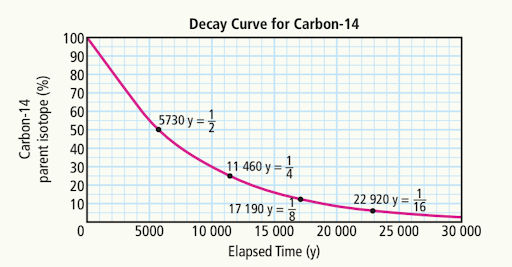
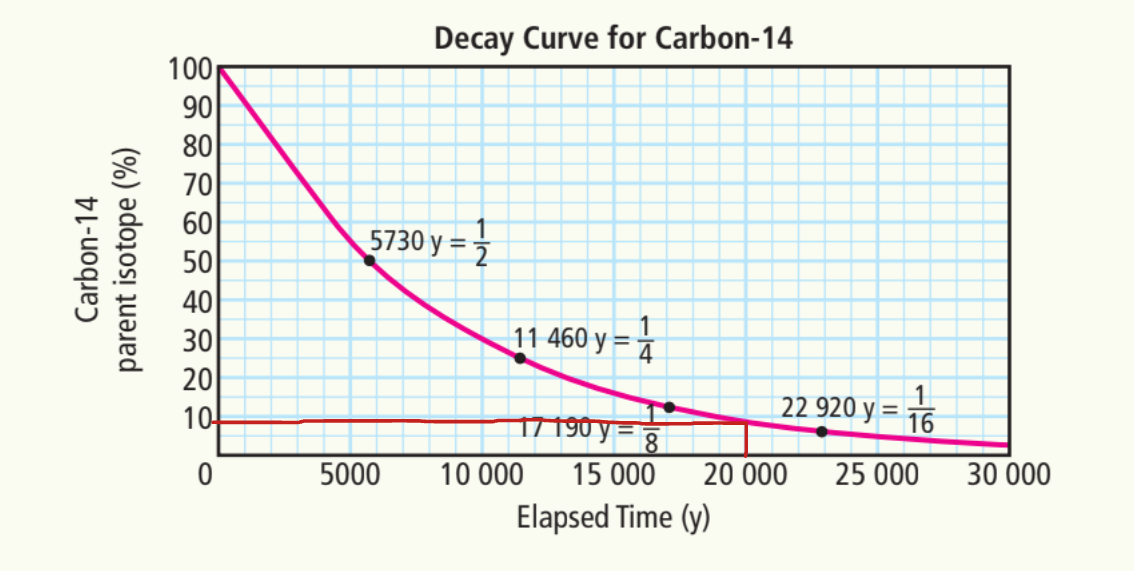
c) 5%
~23,000
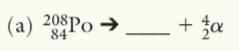
208Pb
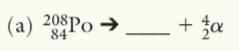
208Pb82
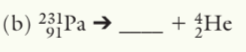
227Ac89
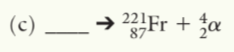
225Ac89
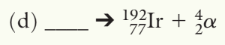
201Tl79
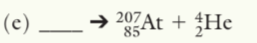
211F87